Strain-specific association of cytotoxic activity and virulence of clinical Staphylococcus aureus isolates
- PMID: 12704146
- PMCID: PMC153241
- DOI: 10.1128/IAI.71.5.2716-2723.2003
Strain-specific association of cytotoxic activity and virulence of clinical Staphylococcus aureus isolates
Abstract
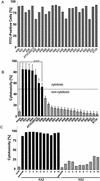
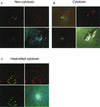
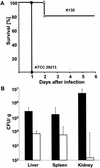
Staphylococcus aureus has been shown to invade and induce the death of various cell types. Here we investigate whether the cytotoxicity of intracellular S. aureus is a general feature or rather characteristic of individual S. aureus strains. The majority of 23 randomly collected clinical S. aureus isolates were killed inside keratinocytes and fibroblasts, indicating that the uptake of S. aureus represents an important mechanism of cell-autonomous host defense. However, seven independent S. aureus isolates survived intracellularly and induced significant cytotoxicity for their host cells. Subcloning analysis revealed that the ability or inability to kill host cells is a stable, apparently genetically determined trait of a given S. aureus isolate. We show that noncytotoxic strains but not cytotoxic strains colocalize with the lysosomal marker LAMP-1, suggesting that only cytotoxic strains escape degradation by the endolysosomal pathway. In a mouse septicemic model, cytotoxic S. aureus isolates produce significantly greater lethality (96%) compared to noncytotoxic strains (41%), which corresponds to 23-, 63-, and 30,000-fold increases of bacterial loads in the liver, spleen, and kidney, respectively. Finally, cytotoxic S. aureus strains produce clinically apparent arthritis in mice at a greater frequency than compared to noncytotoxic S. aureus strains. The results of our study unravel a previously unrecognized dichotomy of cytotoxic and noncytotoxic S. aureus isolates, which may play an important role in the dissemination of, and mortality induced by, S. aureus infection.
Figures



Similar articles
-
Clinical S. aureus Isolates Vary in Their Virulence to Promote Adaptation to the Host.Toxins (Basel). 2019 Mar 1;11(3):135. doi: 10.3390/toxins11030135. Toxins (Basel). 2019. PMID: 30823631 Free PMC article.
-
Genetic diversity and virulence characteristics of Staphylococcus aureus isolates from cases of bovine mastitis.Microb Pathog. 2020 Jul;144:104171. doi: 10.1016/j.micpath.2020.104171. Epub 2020 Mar 26. Microb Pathog. 2020. PMID: 32224210
-
Variation among Staphylococcus aureus membrane vesicle proteomes affects cytotoxicity of host cells.Microb Pathog. 2016 Apr;93:185-93. doi: 10.1016/j.micpath.2016.02.014. Epub 2016 Feb 27. Microb Pathog. 2016. PMID: 26924795
-
Intracellular Staphylococcus aureus employs the cysteine protease staphopain A to induce host cell death in epithelial cells.PLoS Pathog. 2021 Sep 2;17(9):e1009874. doi: 10.1371/journal.ppat.1009874. eCollection 2021 Sep. PLoS Pathog. 2021. PMID: 34473800 Free PMC article.
-
Post-invasion events after infection with Staphylococcus aureus are strongly dependent on both the host cell type and the infecting S. aureus strain.Clin Microbiol Infect. 2016 Sep;22(9):799-809. doi: 10.1016/j.cmi.2016.06.020. Epub 2016 Jul 5. Clin Microbiol Infect. 2016. PMID: 27393124
Cited by
-
Cytoplasmic replication of Staphylococcus aureus upon phagosomal escape triggered by phenol-soluble modulin α.Cell Microbiol. 2014 Apr;16(4):451-65. doi: 10.1111/cmi.12233. Epub 2013 Nov 12. Cell Microbiol. 2014. PMID: 24164701 Free PMC article.
-
Expression of lysostaphin in HeLa cells protects from host cell killing by intracellular Staphylococcus aureus.Med Microbiol Immunol. 2006 Sep;195(3):159-63. doi: 10.1007/s00430-006-0014-1. Epub 2006 Feb 16. Med Microbiol Immunol. 2006. PMID: 16482441
-
Artificial Selection for Pathogenicity Mutations in Staphylococcus aureus Identifies Novel Factors Relevant to Chronic Infection.Infect Immun. 2019 Mar 25;87(4):e00884-18. doi: 10.1128/IAI.00884-18. Print 2019 Apr. Infect Immun. 2019. PMID: 30642903 Free PMC article.
-
Intracellular Staphylococcus aureus Perturbs the Host Cell Ca2+ Homeostasis To Promote Cell Death.mBio. 2020 Dec 15;11(6):e02250-20. doi: 10.1128/mBio.02250-20. mBio. 2020. PMID: 33323513 Free PMC article.
-
SarA based novel therapeutic candidate against Staphylococcus aureus associated with vascular graft infections.Front Microbiol. 2015 May 6;6:416. doi: 10.3389/fmicb.2015.00416. eCollection 2015. Front Microbiol. 2015. PMID: 26074884 Free PMC article.
References
-
- Almeida, R. A., K. R. Matthews, E. Cifrian, A. J. Guidry, and S. P. Oliver. 1996. Staphylococcus aureus invasion of bovine mammary epithelial cells. J. Dairy Sci. 79:1021-1026. - PubMed
-
- Balwit, J. M., P. van Langevelde, J. M. Vann, and R. A. Proctor. 1994. Gentamicin-resistant menadione and hemin auxotrophic Staphylococcus aureus persist within cultured endothelial cells. J. Infect. Dis. 170:1033-1037. - PubMed
-
- Beekhuizen, H., J. S. van de Gevel, B. Olsson, I. J. van Benten, and R. van Furth. 1997. Infection of human vascular endothelial cells with Staphylococcus aureus induces hyperadhesiveness for human monocytes and granulocytes. J. Immunol. 158:774-782. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

