Production of the siderophore 2,3-dihydroxybenzoic acid is required for wild-type growth of Brucella abortus in the presence of erythritol under low-iron conditions in vitro
- PMID: 12704172
- PMCID: PMC153232
- DOI: 10.1128/IAI.71.5.2927-2932.2003
Production of the siderophore 2,3-dihydroxybenzoic acid is required for wild-type growth of Brucella abortus in the presence of erythritol under low-iron conditions in vitro
Abstract
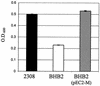
Production of the siderophore 2,3-dihyroxybenzoic acid (2,3-DHBA) is required for the wild-type virulence of Brucella abortus in cattle. A possible explanation for this requirement was uncovered when it was determined that a B. abortus dhbC mutant (BHB1) defective in 2,3-DHBA production displays marked growth restriction in comparison to its parent strain, B. abortus 2308, when cultured in the presence of erythritol under low-iron conditions. This phenotype is not displayed when these strains are cultured under low-iron conditions in the presence of other readily utilizable carbon and energy sources. The addition of either exogenous 2,3-DHBA or FeCl(3) relieves this growth defect, suggesting that the inability of the B. abortus dhbC mutant to display wild-type growth in the presence of erythritol under iron-limiting conditions is due to a defect in iron acquisition. Restoring 2,3-DHBA production to the B. abortus dhbC mutant by genetic complementation abolished the erythritol-specific growth defect exhibited by this strain in low-iron medium, verifying the relationship between 2,3-DHBA production and efficient growth in the presence of erythritol under low-iron conditions. The positive correlation between 2,3-DHBA production and growth in the presence of erythritol was further substantiated by the observation that the addition of erythritol to low-iron cultures of B. abortus 2308 stimulated the production of 2,3-DHBA by increasing the transcription of the dhbCEBA operon. Correspondingly, the level of exogenous iron needed to repress dhbCEBA expression in B. abortus 2308 was also greater when this strain was cultured in the presence of erythritol than that required when it was cultured in the presence of any of the other readily utilizable carbon and energy sources tested. The tissues of the bovine reproductive tract are rich in erythritol during the latter stages of pregnancy, and the ability to metabolize erythritol is thought to be important to the virulence of B. abortus in pregnant ruminants. Consequently, the experimental findings presented here offer a plausible explanation for the attenuation of the B. abortus 2,3-DHBA-deficient mutant BHB1 in pregnant ruminants.
Figures

References
-
- Alexander, B., P. R. Schnurrenberger, and R. R. Brown. 1981. Numbers of Brucella abortus in the placenta, umbilicus and fetal fluid of two naturally infected cows. Vet. Rec. 108:500.. - PubMed
-
- Arnow, L. E. 1937. Colorimetric determination of the components of 3,4-dihydroxyphenyalanin-tyrosine mixtures. J. Biol. Chem. 118:531-537.
-
- Byers, B. R., and J. E. Arceneaux. 1998. Microbial iron transport: iron acquisition by pathogenic microorganisms. Met. Ions Biol. Syst. 35:37-66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

