Toxic epidermal necrolysis and Stevens-Johnson syndrome are induced by soluble Fas ligand
- PMID: 12707034
- PMCID: PMC1851208
- DOI: 10.1016/S0002-9440(10)64284-8
Toxic epidermal necrolysis and Stevens-Johnson syndrome are induced by soluble Fas ligand
Abstract
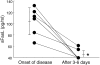
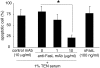
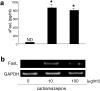
The pathogeneses of toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS), both severe blistering diseases usually associated with drug intake, are not fully elucidated. Histologically, both TEN and SJS are characterized by extensive keratinocyte apoptosis. Previous studies have shown that keratinocyte apoptosis in TEN and SJS was induced by a suicidal interaction between Fas and Fas ligand (FasL), which are both expressed by keratinocytes. However, our preliminary examinations demonstrated that FasL is hardly detected on keratinocytes. We hypothesized that soluble FasL (sFasL) is secreted by peripheral blood mononuclear cells (PBMCs), and this interacts with the Fas expressed on keratinocytes in TEN and SJS. To justify this hypothesis, we investigated whether sFasL secreted by PBMCs could induce the keratinocyte apoptosis in TEN and SJS. Enzyme-linked immunosorbent assay analysis demonstrated that there was no significant sFasL increase in any samples of healthy controls (<40 pg/ml, n = 14) and patients with an ordinary erythema multiforme-type drug eruption (41.5 +/- 3.1 pg/ml, n = 14), whereas high concentrations are detected in all samples of TEN and SJS patients (TEN: 131.5 +/- 57.4 pg/ml, n = 8; SJS: 119.1 +/- 41.0 pg/ml, n = 14) (P < 0.0001). In vitro analysis using cultured keratinocytes revealed that the sera of TEN and SJS patients induced abundant keratinocyte apoptosis compared to erythema multiforme-type drug eruption sera. Furthermore, on stimulation with the causal drug, PBMCs obtained from TEN and SJS patients secreted high levels of sFasL. Taken together, these results indicate that sFasL secreted by PBMCs, not keratinocytes, plays a crucial role in the apoptosis and pathomechanism of TEN and SJS, and that the serum sFasL level may be a good indicator for the early diagnosis of TEN and SJS.
Figures



References
-
- Becker DS: Toxic epidermal necrolysis. Lancet 1998, 351:1417-1420 - PubMed
-
- Roujeau JC: The spectrum of Stevens-Johnson syndrome and toxic epidermal necrolysis: a clinical classification. J Invest Dermatol 1994, 102:28-30 - PubMed
-
- Wolkenstein PC, Adle H, Wechsler J, Garchon HJ, Revuz J, Roujeau JC: Apoptosis as a mechanism of keratinocyte death in toxic epidermal necrolysis. Br J Dermatol 1996, 134:710-714 - PubMed
-
- Correia O, Delgado L, Ramos JP, Resende C, Torrinha JA: Cutaneous T-cell recruitment in toxic epidermal necrolysis. Further evidence of CD8+ lymphocyte involvement. Arch Dermatol 1993, 129:466-468 - PubMed
-
- Wolkenstein P, Charue D, Laurent P, Revuz J, Roujeau JC, Bagot M: Metabolic predisposition to cutaneous adverse drug reactions. Role in toxic epidermal necrolysis caused by sulfonamides and anticonvulsants. Arch Dermatol 1995, 131:544-551 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

