Identification and characterization of the hepatic stellate cell transferrin receptor
- PMID: 12707050
- PMCID: PMC1851195
- DOI: 10.1016/S0002-9440(10)64300-3
Identification and characterization of the hepatic stellate cell transferrin receptor
Abstract
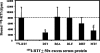
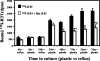
Activated hepatic stellate cells have been implicated in the fibrogenic process associated with iron overload, both in animal models and in human hemochromatosis. Previous studies have evaluated the role of ferritin/ferritin receptor interactions in the activation of stellate cells and subsequent fibrogenesis; however, the role of transferrin in hepatic stellate cell biology is unknown. This study was designed to identify and characterize the stellate cell transferrin receptor and to evaluate the influence of transferrin on stellate cell activation. Identification and characterization of the stellate cell transferrin receptor was determined by competitive displacement assays. The effect of transferrin on stellate cell activation was assessed using western blot analysis for alpha-smooth muscle actin expression, [(3)H]Thymidine incorporation, and real-time RT-PCR for procollagen alpha1(I) mRNA expression. A specific receptor for rat transferrin was observed on activated but not quiescent stellate cells. Transferrin significantly increased the expression of alpha-smooth muscle actin, but caused a decrease in proliferation. Transferrin induced a significant increase in procollagen alpha1(I) mRNA expression. In conclusion, this study has demonstrated for the first time a specific, high affinity receptor for rat transferrin on activated hepatic stellate cells, which via interaction with transferrin regulates stellate cell activation. This suggests that transferrin may be an important factor in the activation of hepatic stellate cells in conditions of iron overload.
Figures





References
-
- Friedman SL: Hepatic stellate cells. Prog Liver Dis 1996, 14:101-130 - PubMed
-
- Ooi LP, Crawford DH, Gotley DC, Clouston AD, Strong RW, Gobe GC, Halliday JW, Bridle KR, Ramm GA: Evidence that “myofibroblast-like” cells are the cellular source of capsular collagen in hepatocellular carcinoma. J Hepatol 1997, 26:798-807 - PubMed
-
- Ramm GA, Crawford DH, Powell LW, Walker NI, Fletcher LM, Halliday JW: Hepatic stellate cell activation in genetic haemochromatosis. Lobular distribution, effect of increasing hepatic iron and response to phlebotomy. J Hepatol 1997, 26:584-592 - PubMed
-
- Ramm GA, Li SC, Li L, Britton RS, O’Neill R, Kobayashi Y, Bacon BR: Chronic iron overload causes activation of rat lipocytes in vivo. Am J Physiol 1995, 268:G451-G458 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

