Human dendritic cells activated by TSLP and CD40L induce proallergic cytotoxic T cells
- PMID: 12707303
- PMCID: PMC2193883
- DOI: 10.1084/jem.20030240
Human dendritic cells activated by TSLP and CD40L induce proallergic cytotoxic T cells
Abstract
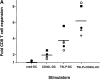
Human thymic stromal lymphopoietin (TSLP) is a novel epithelial cell-derived cytokine, which induces dendritic cell (DC)-mediated CD4+ T cell responses with a proallergic phenotype. Although the participation of CD8+ T cells in allergic inflammation is well documented, their functional properties as well as the pathways leading to their generation remain poorly understood. Here, we show that TSLP-activated CD11c+ DCs potently activate and expand naive CD8+ T cells, and induce their differentiation into interleukin (IL)-5 and IL-13-producing effectors exhibiting poor cytolytic activity. Additional CD40L triggering of TSLP-activated DCs induced CD8+ T cells with potent cytolytic activity, producing large amounts of interferon (IFN)-gamma, while retaining their capacity to produce IL-5 and IL-13. These data further support the role of TSLP as initial trigger of allergic T cell responses and suggest that CD40L-expressing cells may act in combination with TSLP to amplify and sustain pro-allergic responses and cause tissue damage by promoting the generation of IFN-gamma-producing cytotoxic effectors.
Figures

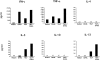


References
-
- Reche, P.A., V. Soumelis, D.M. Gorman, T. Clifford, M. Liu, M. Travis, S.M. Zurawski, J. Johnston, Y.J. Liu, H. Spits, et al. 2001. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J. Immunol. 167:336–343. - PubMed
-
- Soumelis, V., P.A. Reche, H. Kanzler, W. Yuan, G. Edward, B. Homey, M. Gilliet, S. Ho, S. Antonenko, A. Lauerma, et al. 2002. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 3:673–680. - PubMed
-
- Kay, A.B. 2001. Allergy and allergic diseases. First of two parts. N. Engl. J. Med. 344:30–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

