Regulation of beta cell glucokinase by S-nitrosylation and association with nitric oxide synthase
- PMID: 12707306
- PMCID: PMC2172922
- DOI: 10.1083/jcb.200301063
Regulation of beta cell glucokinase by S-nitrosylation and association with nitric oxide synthase
Abstract
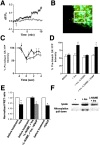
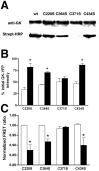
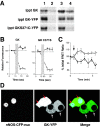
Glucokinase (GK) activity plays a key role in glucose-stimulated insulin secretion from pancreatic beta cells. Insulin regulates GK activity by modulating its association with secretory granules, although little is known about the mechanisms involved in regulating this association. Using quantitative imaging of multicolor fluorescent proteins fused to GK, we found that the dynamic association of GK with secretory granules is modulated through nitric oxide (NO). Our results in cultured beta cells show that insulin stimulates NO production and leads to S-nitrosylation of GK. Furthermore, inhibition of NO synthase (NOS) activity blocks insulin-stimulated changes in both GK association with secretory granules and GK conformation. Mutation of cysteine 371 to serine blocks S-nitrosylation of GK and causes GK to remain tightly bound to secretory granules. GK was also found to interact stably with neuronal NOS as detected by coimmunoprecipitation and fluorescence resonance energy transfer. Finally, attachment of a nuclear localization signal sequence to NOS drives GK to the nucleus in addition to its normal cytoplasmic and granule targeting. Together, these data suggest that the regulation of GK localization and activity in pancreatic beta cells is directly related to NO production and that the association of GK with secretory granules occurs through its interaction with NOS.
Figures
References
-
- Aspinwall, C.A., W.J. Qian, M.G. Roper, R.N. Kulkarni, C.R. Kahn, and R.T. Kennedy. 2000. Roles of insulin receptor substrate-1, phosphatidylinositol 3-kinase, and release of intracellular Ca2+ stores in insulin-stimulated insulin secretion in β-cells. J. Biol. Chem. 275:22331–22338. - PubMed
-
- Brenman, J.E., D.S. Chao, S.H. Gee, A.W. McGee, S.E. Craven, D.R. Santillano, Z. Wu, F. Huang, H. Xia, M.F. Peters, et al. 1996. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 84:757–767. - PubMed
-
- Fang, M., S.R. Jaffrey, A. Sawa, K. Ye, X. Luo, and S.H. Snyder. 2000. Dexras1: a G protein specifically coupled to neuronal nitric oxide synthase via CAPON. Neuron. 28:183–193. - PubMed
-
- Henningsson, R., A. Salehi, and I. Lundquist. 2002. Role of nitric oxide synthase isoforms in glucose-stimulated insulin release. Am. J. Physiol. Cell Physiol. 283:C296–C304. - PubMed
-
- Jaffrey, S.R., H. Erdjument-Bromage, C.D. Ferris, P. Tempst, and S.H. Snyder. 2001. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat. Cell Biol. 3:193–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

