Aminoglycoside resistance resulting from tight drug binding to an altered aminoglycoside acetyltransferase
- PMID: 12709325
- PMCID: PMC153337
- DOI: 10.1128/AAC.47.5.1577-1583.2003
Aminoglycoside resistance resulting from tight drug binding to an altered aminoglycoside acetyltransferase
Abstract
The aacA29b gene, which confers an atypical aminoglycoside resistance pattern to Escherichia coli, was identified on a class 1 integron from a multidrug-resistant isolate of Pseudomonas aeruginosa. On the basis of amino acid sequence homology, it was proposed that the gene encoded a 6'-N-acetyltransferase. The resistance gene was cloned into the pET23a(+) vector, and overexpression conferred high-level resistance to the usual substrates of the aminoglycoside N-acetyltransferase AAC(6')-I, except netilmicin. The level of resistance conferred by aacA29b correlated perfectly with the level of expression of the gene. The corresponding C-terminal six-His-tagged AAC(6')-29b protein was purified and found to exist as a dimer in solution. With a spectrophotometric assay, an extremely feeble AAC activity was detected with acetyl coenzyme A (acetyl-CoA) as an acetyl donor. Fluorescence titrations of the protein with aminoglycosides demonstrated the very tight binding of tobramycin, dibekacin, kanamycin A, sisomicin (K(d), </=1 micro M) and a weaker affinity for amikacin (K(d), approximately 60 micro M). The binding of netilmicin and acetyl-CoA could not be detected by either fluorescence spectroscopy or isothermal titration calorimetry. The inability of AAC(6')-29b to efficiently bind acetyl-CoA is supported by an alignment analysis of its amino acid sequence compared with those of other AAC(6')-I family members. AAC(6')-29b lacks a number of residues involved in acetyl-CoA binding. These results lead to the conclusion that AAC(6')-29b is able to confer aminoglycoside resistance by sequestering the drug as a result of tight binding.
Figures


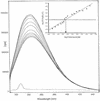
References
-
- Chipman, D. M., V. Grisaro, and N. Sharon. 1967. The binding of oligosaccharides containing N-acetylglucosamine and N-acetylmuramic acid to lysozyme. The specificity of binding subsites. J. Biol. Chem. 242:4388-4394. - PubMed
-
- Davies, J., and G. D. Wright. 1997. Bacterial resistance to aminoglycoside antibiotics. Trends Microbiol. 5:234-240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

