Adenosine 5'-triphosphate (ATP) is an excitatory cotransmitter with noradrenaline to the smooth muscle of the rat prostate gland
- PMID: 12711628
- PMCID: PMC1573777
- DOI: 10.1038/sj.bjp.0705167
Adenosine 5'-triphosphate (ATP) is an excitatory cotransmitter with noradrenaline to the smooth muscle of the rat prostate gland
Abstract
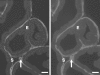
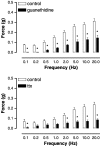
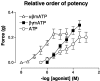
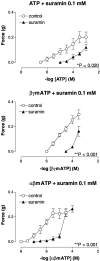
1. This study investigated whether adenosine 5'-triphosphate (ATP) is involved in neurotransmission to the rat prostate gland. 2. Fluorescence immunohistochemistry carried out on formaldehyde-fixed and frozen sections of rat prostate showed immunoreactivity for the P2X(1)-receptor in the fibromuscular stroma surrounding the secretory acini but not in the glandular epithelium. P2X(2)-, P2X(3)-, P2X(4)- and P2X(7)-receptors were immunonegative in the rat prostate stroma. Double-staining procedures showed P2X(1)-receptor immunoreactivity to be colocalized with alpha-actin immunoreactivity. 3. Isolated organ bath studies investigated whether drugs, which modify purinergic mechanisms, are able to affect contractility of the rat prostate gland. Suramin (100 micro M) and alphabetamethylene ATP (10 micro M) inhibited contractile responses to trains of electrical-field stimulation (70 V, 0.5 ms, 0.1-2 Hz) in the absence and presence of prazosin (300 nM). Responses to 5-20 Hz were unaffected by suramin or alphabetamethylene ATP. 4. Exogenous application of ATP analogues to unstimulated isolated preparations of rat prostate produced concentration-dependent suramin (100 micro M) sensitive transient contractions with a relative order of potency: alphabetamethylene ATP>betagammamethylene ATP>ATP. 5. Adenosine and adenosine 5'-monophosphate (AMP) did not produce contractile responses. 6. These results suggest that P2X(1)-receptors for ATP, which mediate contractions are present in the fibromuscular stroma of the rat prostate. The relative order of potency of ATP analogues in producing contractions of the rat prostate is consistent with the activation of P2X(1)-receptors. Inhibition by suramin and alphabetamethylene ATP of electrically evoked nerve-mediated contractions of the rat prostate implies that ATP contributes to this contractile response and is therefore a cotransmitter with noradrenaline during low-frequency stimulation.
Figures


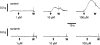
Similar articles
-
Adenosine 5'-triphosphate and noradrenaline are excitatory cotransmitters to the fibromuscular stroma of the guinea pig prostate gland.Eur J Pharmacol. 2004 Sep 24;499(3):335-44. doi: 10.1016/j.ejphar.2004.07.080. Eur J Pharmacol. 2004. PMID: 15381056
-
Stimulation of lumbar sympathetic nerves evokes contractions of cat colon circular muscle mediated by ATP and noradrenaline.Br J Pharmacol. 1993 Nov;110(3):1260-70. doi: 10.1111/j.1476-5381.1993.tb13951.x. Br J Pharmacol. 1993. PMID: 7905343 Free PMC article.
-
Effects of adenine nucleosides and nucleotides on neuromuscular transmission to the prostatic stroma of the rat.Br J Pharmacol. 2000 Nov;131(6):1073-80. doi: 10.1038/sj.bjp.0703652. Br J Pharmacol. 2000. PMID: 11082113 Free PMC article.
-
ATP as a cotransmitter in perivascular sympathetic nerves.J Auton Pharmacol. 1996 Dec;16(6):337-40. doi: 10.1111/j.1474-8673.1996.tb00048.x. J Auton Pharmacol. 1996. PMID: 9131411 Review.
-
Purinergic signalling in endocrine organs.Purinergic Signal. 2014 Mar;10(1):189-231. doi: 10.1007/s11302-013-9396-x. Epub 2013 Nov 22. Purinergic Signal. 2014. PMID: 24265070 Free PMC article. Review.
Cited by
-
Age-related changes in the innervation of the prostate gland: implications for prostate cancer initiation and progression.Organogenesis. 2013 Jul-Sep;9(3):206-15. doi: 10.4161/org.24843. Epub 2013 May 14. Organogenesis. 2013. PMID: 23872639 Free PMC article. Review.
-
Characterisation of the prostanoid receptor mediating inhibition of smooth muscle contractility in the rat prostate gland.Naunyn Schmiedebergs Arch Pharmacol. 2010 Apr;381(4):321-8. doi: 10.1007/s00210-010-0492-y. Epub 2010 Feb 24. Naunyn Schmiedebergs Arch Pharmacol. 2010. PMID: 20180098
-
Novel drug targets for the pharmacotherapy of benign prostatic hyperplasia (BPH).Br J Pharmacol. 2011 Jul;163(5):891-907. doi: 10.1111/j.1476-5381.2011.01332.x. Br J Pharmacol. 2011. PMID: 21410684 Free PMC article. Review.
-
Induction of chronic prostatitis does not alter the innate contractile properties of the prostate or urethra in rats.Sci Rep. 2025 Jun 20;15(1):20226. doi: 10.1038/s41598-025-06531-7. Sci Rep. 2025. PMID: 40542053 Free PMC article.
-
Stimulation of epithelial CB1 receptors inhibits contractions of the rat prostate gland.Br J Pharmacol. 2007 Jan;150(2):227-34. doi: 10.1038/sj.bjp.0706952. Epub 2006 Nov 13. Br J Pharmacol. 2007. PMID: 17099718 Free PMC article.
References
-
- ABBRACCHIO M.P., BURNSTOCK G. Purinergic signalling: pathophysiological roles. Jpn. J. Pharmacol. 1998;78:113–145. - PubMed
-
- BO X., BURNSTOCK G. Species differences in characteristics and distribution of [3H]α,βmethylene ATP binding sites in urinary bladder and urethra of rat, guinea-pig and rabbit. Eur. J. Pharmacol. 1992;216:59–66. - PubMed
-
- BO X., BURNSTOCK G. Heterogeneous distribution of [3H]α,βmethylene ATP binding sites in blood vessels. J. Vasc. Res. 1993;30:87–101. - PubMed
-
- BURNSTOCK G.A basis for distinguishing two types of purinergic receptor Cell Membrane Receptors for Drugs and Hormones: A Multidisciplinary Approach 1978New York: Raven Press; 107–118.eds. Straub, R.W. & Bolis, L
-
- BURNSTOCK G., KENNEDY C. Is there a basis for distinguishing two types of P2-purinoceptor. Gen. Pharmacol. 1985;16:433–440. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

