Vasodilator actions of abnormal-cannabidiol in rat isolated small mesenteric artery
- PMID: 12711633
- PMCID: PMC1573773
- DOI: 10.1038/sj.bjp.0705160
Vasodilator actions of abnormal-cannabidiol in rat isolated small mesenteric artery
Abstract
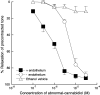
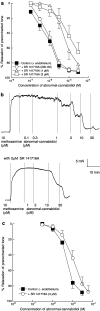
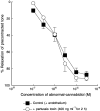
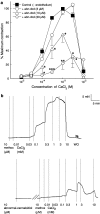
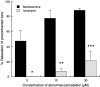
1. The nonpsychoactive cannabinoid abnormal-cannabidiol (trans-4-[3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-5-pentyl-1,3-benzenediol) (abn-cbd) produced concentration-dependent relaxation of methoxamine-precontracted rat small mesenteric artery. Endothelial removal reduced abn-cbd potency six-fold without affecting the maximum relaxation. 2. In endothelium-intact vessels, abn-cbd was less potent under 60 mM KCl-induced tone and inhibited by combination of L-N(G)-nitroarginine methyl ester (L-NAME) (nitric oxide synthase inhibitor; 300 micro M), apamin (small conductance Ca(2+)-activated K(+) channels inhibitor; 50 nM) and charybdotoxin (inhibitor of intermediate conductance Ca(2+)-activated K(+) channels and large conductance Ca(2+)-activated K(+) channels BK(Ca); 50 nM). L-NAME alone or in combination with either toxin alone had little effect. 3. In intact vessels, relaxations to abn-cbd were inhibited by SR 141716A (cannabinoid receptor antagonist; 1 or 3 micro M). Concomitant addition of L-NAME, apamin and charybdotoxin had no further effect. Other cannabinoid receptor antagonists either had little (SR 144528; 1 micro M and AM 251; 1 micro M) or no effect (AM 630; 10 micro M and AM 281; 1 micro M). Inhibition of gap junctions, G(i/o) protein coupling and protein kinase A also had no effect. 4. Endothelium-independent relaxation to abn-cbd was unaffected by L-NAME, apamin plus charybdotoxin or capsaicin (10 micro M). Abn-cbd inhibited CaCl(2)-induced contractions in vessels with depleted intracellular Ca(2+) stores and stimulated with methoxamine or KCl. This was insensitive to SR 141716A (3 micro M) but greatly reduced in vessels stimulated with ionomycin (Ca(2+) ionophore; 1 micro M). 5. We conclude that abn-cbd relaxes the rat small mesenteric artery by endothelium-dependent activation of K(+) channels via SR 141716A-sensitive pathways, which do not involve CB(1) and CB(2) receptors. It also causes endothelium-independent, SR 141716A-insensitive, relaxation by inhibiting Ca(2+) entry through voltage-gated Ca(2+) channels.
Figures



Similar articles
-
Endothelium-independent relaxation to cannabinoids in rat-isolated mesenteric artery and role of Ca2+ influx.Br J Pharmacol. 2003 Jun;139(3):585-97. doi: 10.1038/sj.bjp.0705280. Br J Pharmacol. 2003. PMID: 12788818 Free PMC article.
-
A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery.Br J Pharmacol. 1997 Dec;122(8):1573-84. doi: 10.1038/sj.bjp.0701546. Br J Pharmacol. 1997. PMID: 9422801 Free PMC article.
-
The actions of the cannabinoid receptor antagonist, SR 141716A, in the rat isolated mesenteric artery.Br J Pharmacol. 1998 Oct;125(4):689-96. doi: 10.1038/sj.bjp.0702127. Br J Pharmacol. 1998. PMID: 9831903 Free PMC article.
-
Endothelial modulation of agonist-induced vasoconstriction in mesenteric microcirculation.Yakugaku Zasshi. 2010 May;130(5):723-8. doi: 10.1248/yakushi.130.723. Yakugaku Zasshi. 2010. PMID: 20460871 Review.
-
Endothelial atypical cannabinoid receptor: do we have enough evidence?Br J Pharmacol. 2014 Dec;171(24):5573-88. doi: 10.1111/bph.12866. Br J Pharmacol. 2014. PMID: 25073723 Free PMC article. Review.
Cited by
-
Minireview: recent developments in the physiology and pathology of the lysophosphatidylinositol-sensitive receptor GPR55.Mol Endocrinol. 2011 Nov;25(11):1835-48. doi: 10.1210/me.2011-1197. Epub 2011 Sep 29. Mol Endocrinol. 2011. PMID: 21964594 Free PMC article. Review.
-
Endo-cannabinoids system and the toxicity of cannabinoids with a biotechnological approach.EXCLI J. 2017 May 15;16:688-711. doi: 10.17179/excli2017-257. eCollection 2017. EXCLI J. 2017. PMID: 28827985 Free PMC article. Review.
-
Blood pressure regulation by endocannabinoids and their receptors.Neuropharmacology. 2005 Jun;48(8):1130-8. doi: 10.1016/j.neuropharm.2004.12.005. Epub 2005 Feb 19. Neuropharmacology. 2005. PMID: 15910888 Free PMC article. Review.
-
The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease.Int J Mol Sci. 2020 Sep 14;21(18):6740. doi: 10.3390/ijms21186740. Int J Mol Sci. 2020. PMID: 32937917 Free PMC article. Review.
-
Endothelium-independent relaxation to cannabinoids in rat-isolated mesenteric artery and role of Ca2+ influx.Br J Pharmacol. 2003 Jun;139(3):585-97. doi: 10.1038/sj.bjp.0705280. Br J Pharmacol. 2003. PMID: 12788818 Free PMC article.
References
-
- ADAMS M.D., EARNHARDT J.T., MARTIN B.R., HARRIS L.S., DEWEY W.L., RAZDAN R.K. A cannabinoid with cardiovascular activity but no overt behavioural effects. Experientia. 1977;33:1204–1205. - PubMed
-
- AGURELL S., HALLDIN M., LINDGREN J.E., OHLSSON A., WIDMAN M., GILLESPIE H., HOLLISTER L. Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol. Rev. 1986;38:21–43. - PubMed
-
- BENOWITZ N.L., JONES R.T. Cardiovascular effects of prolonged delta-9-tetrahydro-cannabinol ingestion. Clin. Pharmacol. Ther. 1974;18:287–297. - PubMed
-
- BISOGNO T., HANUS L., DE PETROCELLIS L., TCHILIBON S., PONDE D.E., BRANDI I., MORIELLO A.S., DAVIS J.B., MECHOULAM R., DI MARZO V. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001;134:845–852. - PMC - PubMed
-
- BREIVOGEL C.S., GRIFFIN G., DI MARZO V., MARTIN B.R. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol. Pharmacol. 2001;60:155–163. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

