Evidence for cross-talk between M2 and M3 muscarinic acetylcholine receptors in the regulation of second messenger and extracellular signal-regulated kinase signalling pathways in Chinese hamster ovary cells
- PMID: 12711635
- PMCID: PMC1573780
- DOI: 10.1038/sj.bjp.0705178
Evidence for cross-talk between M2 and M3 muscarinic acetylcholine receptors in the regulation of second messenger and extracellular signal-regulated kinase signalling pathways in Chinese hamster ovary cells
Abstract
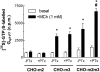
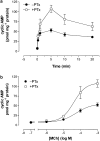
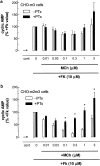
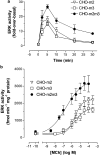
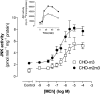
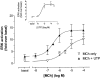
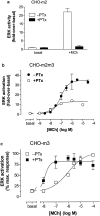
1. We have examined possible mechanisms of cross-talk between the G(q/11)-linked M(3) muscarinic acetylcholine (mACh) receptor and the G(i/o)-linked M(2) mACh receptor by stable receptor coexpression in Chinese hamster ovary (CHO) cells. A number of second messenger (cyclic AMP, Ins(1,4,5)P(3)) and mitogen-activated protein kinase (ERK and JNK) responses stimulated by the mACh receptor agonist methacholine were examined in CHO-m2m3 cells and compared to those stimulated in CHO-m2 and CHO-m3 cell-lines, expressing comparable levels of M(2) or M(3) mACh receptors. 2. Based on comparisons between cell-lines and pertussis toxin (PTx) pretreatment to eliminate receptor-G(i/o) coupling, evidence was obtained for (i) an M(2) mACh receptor-mediated contribution to the predominantly M(3) mACh receptor-mediated Ins(1,4,5)P(3) response and (ii) a facilitation of the inhibitory effect of M(2) mACh receptor on forskolin-stimulated cyclic AMP accumulation by M(3) mACh receptor coactivation at low agonist concentrations (MCh 10(-9)-10(-6) M). 3. The most profound cross-talk effects were observed with respect to ERK activation. Thus, while MCh stimulated ERK activation in both CHO-m2 and CHO-m3 cells (pEC(50) values: 5.64+/-0.09 and 5.57+/-0.16, respectively), the concentration-effect relation was approx 50-fold left-shifted in CHO-m2m3 cells (pEC(50): 7.17+/-0.07). In addition, the ERK response was greater and more sustained in CHO-m2m3 cells. In contrast, only minor differences were seen in the time-courses and concentration-dependencies of JNK activation in CHO-m3 and CHO-m2m3 cells. 4. Costimulation of endogenous P2Y(2) purinoceptors also caused an approx 10-fold left-shift in the MCh-stimulated ERK response in CHO-m2 cells, suggesting that the G(q/11)/G(i/o) interaction to affect ERK activation is not specific to muscarinic receptors. 5. PTx pretreatment of cells had unexpected effects on ERK activation by MCh in both CHO-m2m3 and CHO-m3 cells. Thus, in CHO-m3 cells PTx pretreatment caused a marked left-shift in the MCh concentration-effect curve, while in PTx-treated CHO-m2m3 cells the maximal responsiveness was decreased, but the potency of MCh was only slightly affected. 6. The data presented here strongly suggest that cross-talk between M(2) and M(3) mACh receptors occurs at the level of both second messenger and ERK regulation. Further, these data provide novel insights into the involvement of G(i/o) proteins in both positive and negative modulation of ERK responses evoked by G protein-coupled receptors.
Figures


Similar articles
-
An investigation of whether agonist-selective receptor conformations occur with respect to M2 and M4 muscarinic acetylcholine receptor signalling via Gi/o and Gs proteins.Br J Pharmacol. 2005 Feb;144(4):566-75. doi: 10.1038/sj.bjp.0706090. Br J Pharmacol. 2005. PMID: 15655507 Free PMC article.
-
Regulation of extracellular-signal regulated kinase and c-Jun N-terminal kinase by G-protein-linked muscarinic acetylcholine receptors.Biochem J. 1999 Mar 15;338 ( Pt 3)(Pt 3):619-28. Biochem J. 1999. PMID: 10051431 Free PMC article.
-
G(q/11) and G(i/o) activation profiles in CHO cells expressing human muscarinic acetylcholine receptors: dependence on agonist as well as receptor-subtype.Br J Pharmacol. 2001 Feb;132(4):950-8. doi: 10.1038/sj.bjp.0703892. Br J Pharmacol. 2001. PMID: 11181437 Free PMC article.
-
Pharmacological analysis of the contractile role of M2 and M3 muscarinic receptors in smooth muscle.Recept Channels. 2003;9(4):261-77. Recept Channels. 2003. PMID: 12893538 Review.
-
Mechanisms of cross-talk between G-protein-coupled receptors.Neurosignals. 2002 Jan-Feb;11(1):45-57. doi: 10.1159/000057321. Neurosignals. 2002. PMID: 11943882 Review.
Cited by
-
Acetylcholine-dependent upregulation of TASK-1 channels in thalamic interneurons by a smooth muscle-like signalling pathway.J Physiol. 2017 Sep 1;595(17):5875-5893. doi: 10.1113/JP274527. Epub 2017 Aug 3. J Physiol. 2017. PMID: 28714121 Free PMC article.
-
An investigation of whether agonist-selective receptor conformations occur with respect to M2 and M4 muscarinic acetylcholine receptor signalling via Gi/o and Gs proteins.Br J Pharmacol. 2005 Feb;144(4):566-75. doi: 10.1038/sj.bjp.0706090. Br J Pharmacol. 2005. PMID: 15655507 Free PMC article.
-
Interplay between serotonin 5-HT1A and 5-HT7 receptors in depressive disorders.CNS Neurosci Ther. 2014 Jul;20(7):582-90. doi: 10.1111/cns.12247. CNS Neurosci Ther. 2014. PMID: 24935787 Free PMC article. Review.
-
Luciferase reporter gene assay on human, murine and rat histamine H4 receptor orthologs: correlations and discrepancies between distal and proximal readouts.PLoS One. 2013 Sep 2;8(9):e73961. doi: 10.1371/journal.pone.0073961. eCollection 2013. PLoS One. 2013. PMID: 24023919 Free PMC article.
-
Interaction of P2 purinergic receptors with cellular macromolecules.Naunyn Schmiedebergs Arch Pharmacol. 2008 Mar;377(1):1-33. doi: 10.1007/s00210-007-0222-2. Epub 2007 Dec 19. Naunyn Schmiedebergs Arch Pharmacol. 2008. PMID: 18273661 Free PMC article. Review.
References
-
- ALBERT P.R., ROBILLARD L. G protein specificity: traffic direction required. Cell. Signal. 2002;14:407–418. - PubMed
-
- BATCHELOR A.M., GARTHWAITE J. Frequency detection and temporally dispersed synaptic signal association through a metabotropic glutamate receptor pathway. Nature. 1997;385:74–77. - PubMed
-
- BONNER T.I., BUCKLEY N.J., YOUNG A.C., BRANN M.R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987;237:527–532. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

