Steady-state and time-resolved fluorescence studies indicate an unusual conformation of 2-aminopurine within ATAT and TATA duplex DNA sequences
- PMID: 12711677
- PMCID: PMC154225
- DOI: 10.1093/nar/gkg339
Steady-state and time-resolved fluorescence studies indicate an unusual conformation of 2-aminopurine within ATAT and TATA duplex DNA sequences
Abstract
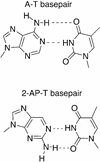
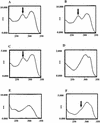
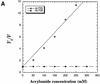
2-Aminopurine (2-AP), a fluorescent analog of adenine, has been widely used as a probe for local DNA conformation, since excitation and emission characteristics and fluorescence lifetimes of 2-AP vary in a sequence-dependent manner within DNA. Using steady-state and time-resolved fluorescence techniques, we report that 2-AP appears to be unusually stacked in the internal positions of ATAT and TATA in duplex DNA. The excitation wavelength maxima for 2-AP within these contexts were red shifted, indicating reduced solvent exposure for the fluorophore. Furthermore, in these contexts, 2-AP fluorescence was resistant to acrylamide-dependent collisional quenching, suggesting that the fluorophore is protected by its stacked position within the duplex. This conclusion was further reinforced by the presence of a secondary peak at 275 nm in the fluorescence excitation spectra that is indicative of efficient excitation energy transfer from nearby non-fluorescent DNA bases. Fluorescence anisotropy decay and internal angular 'wobbling' motion measurements of 2-AP within these alternating AT contexts were also consistent with the fluorophore being highly constrained and immobile within the base stack. When these fluorescence characteristics are compared with those of 2-AP within other duplex DNA sequence contexts, they are unique.
Figures


References
-
- Rachofsky E.L., Osman,R. and Ross,J.B.A. (2001) Probing structure and dynamics of DNA with 2-aminopurine: effects of local environment on fluorescence. Biochemistry, 40, 946–956. - PubMed
-
- Rachofsky E.L., Seibert,E., Stivers,J.T., Osman,R. and Ross,J.B.A. (2001) Conformation and dynamics of abasic sites in DNA investigated by time-resolved fluorescence of 2-aminopurine. Biochemistry, 40, 957–967. - PubMed

