DNA relaxation by human topoisomerase I occurs in the closed clamp conformation of the protein
- PMID: 12711735
- PMCID: PMC156254
- DOI: 10.1073/pnas.1031537100
DNA relaxation by human topoisomerase I occurs in the closed clamp conformation of the protein
Abstract
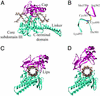
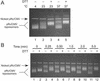
In cocrystal structures of human topoisomerase I and DNA, the enzyme is tightly clamped around the DNA helix. After cleavage and covalent attachment of the enzyme to the 3' end at the nick, DNA relaxation requires rotation of the DNA helix downstream of the cleavage site. Models based on the cocrystal structure reveal that there is insufficient space in the protein for such DNA rotation without some deformation of the cap and linker regions of the enzyme. Alternatively, it is conceivable that the protein clamp opens to facilitate the rotation process. To distinguish between these two possibilities, we engineered two cysteines into the opposing loops of the "lips" region of the enzyme, which allowed us to lock the protein via a disulfide crosslink in the closed conformation around the DNA. Importantly, the rate of DNA relaxation when the enzyme was locked on the DNA was comparable to that observed in the absence of the disulfide crosslink. These results indicate that DNA relaxation likely proceeds without extensive opening of the enzyme clamp.
Figures



References
-
- Champoux J J. In: DNA Topology and Its Biological Effects. Wang J C, Cozzarelli N R, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1990. pp. 217–242.
-
- Wang J C. Annu Rev Biochem. 1996;65:635–692. - PubMed
-
- Champoux J J. Annu Rev Biochem. 2001;70:369–413. - PubMed
-
- Wang J C. Nat Rev Mol Cell Biol. 2002;3:430–440. - PubMed
-
- Stewart L, Redinbo M R, Qiu X, Hol W G, Champoux J J. Science. 1998;279:1534–1541. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

