Efficiency of lignin biosynthesis: a quantitative analysis
- PMID: 12714366
- PMCID: PMC4242356
- DOI: 10.1093/aob/mcg073
Efficiency of lignin biosynthesis: a quantitative analysis
Abstract
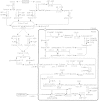
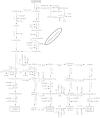
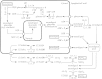
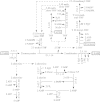
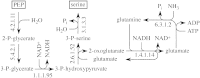
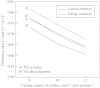
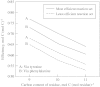
Lignin is derived mainly from three alcohol monomers: p-coumaryl alcohol, coniferyl alcohol and sinapyl alcohol. Biochemical reactions probably responsible for synthesizing these three monomers from sucrose, and then polymerizing the monomers into lignin, were analysed to estimate the amount of sucrose required to produce a unit of lignin. Included in the calculations were amounts of respiration required to provide NADPH (from NADP(+)) and ATP (from ADP) for lignin biosynthesis. Two pathways in the middle stage of monomer biosynthesis were considered: one via tyrosine (found in monocots) and the other via phenylalanine (found in all plants). If lignin biosynthesis proceeds with high efficiency via tyrosine, 76.9, 70.4 and 64.3 % of the carbon in sucrose can be retained in the fraction of lignin derived from p-coumaryl alcohol, coniferyl alcohol and sinapyl alcohol, respectively. The corresponding carbon retention values for lignin biosynthesis via phenylalanine are less, at 73.2, 65.7 and 60.7 %, respectively. Energy (i.e. heat of combustion) retention during lignin biosynthesis via tyrosine could be as high as 81.6, 74.5 and 67.8 % for lignin derived from p-coumaryl alcohol, coniferyl alcohol and sinapyl alcohol, respectively, with the corresponding potential energy retention values for lignin biosynthesis via phenylalanine being less, at 77.7, 69.5 and 63.9 %, respectively. Whether maximum efficiency occurs in situ is unclear, but these values are targets that can be considered in: (1) plant breeding programmes aimed at maximizing carbon or energy retention from photosynthate; (2) analyses of (minimum) metabolic costs of responding to environmental change or pest attack involving increased lignin biosynthesis; (3) understanding costs of lignification in older tissues; and (4) interpreting carbon balance measurements of organs and plants with large lignin concentrations.
Figures

References
-
- AgarwalUP, Atalla RH.1986. In‐situ microprobe studies of plant cell walls: macromolecular organization and compositional variability in the secondary wall of Picea mariana (Mill.) B.S.P. Planta 169: 325–332. - PubMed
-
- AmthorJS.1994. Respiration and carbon assimilate use. In: Boote KJ, Bennett JM, Sinclair TR, Paulsen GM, eds. Physiology and determination of crop yield Madison: American Society of Agronomy, 221–250.
-
- AmthorJS.2000. The McCree–de Wit–Penning de Vries–Thornley respiration paradigms: 30 years later. Annals of Botany 86: 1–20.
-
- ap ReesT.1985. The organization of glycolysis and the oxidative pentose phosphate pathway in plants. In: Douce R, Day DA, eds. Encyclopedia of plant physiology, new series, volume 18 Berlin: Springer‐Verlag, 391–417.
-
- ap ReesT.1988. Hexose phosphate metabolism by nonphotosynthetic tissues of higher plants. In: Preiss J, ed. The biochemistry of plants, volume 14 San Diego: Academic Press, 1–33.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

