Ultrasound activates the TM ELAM-1/IL-1/NF-kappaB response: a potential mechanism for intraocular pressure reduction after phacoemulsification
- PMID: 12714632
- PMCID: PMC1950284
- DOI: 10.1167/iovs.02-0631
Ultrasound activates the TM ELAM-1/IL-1/NF-kappaB response: a potential mechanism for intraocular pressure reduction after phacoemulsification
Abstract
Purpose: Elevated intraocular pressure (IOP), the major causal risk factor for glaucoma, often decreases after cataract removal by phacoemulsification ultrasound. In this study, the hypothesis that ultrasound energy propagated through a fluid medium induces a stress response with the potential to lower IOP was investigated.
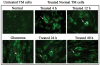
Methods: Normal and glaucomatous trabecular meshwork (TM) cell culture lines were initiated from tissue isolated from human cadaveric eyes or trabeculectomy specimens. Cultured cells were treated for 60 seconds with a phacoemulsification ultrasound probe set to a power of 70%. Activation of the TM cell-specific stress response was assayed by enzyme-linked immunosorbent assay (ELISA) and immunolocalization.
Results: Normal TM cell cultures did not release detectable levels of the stress response protein, IL-1alpha, into their culture medium. In contrast, IL-1alpha was easily detected after treatment with ultrasound energy. Consistent with earlier findings, glaucomatous TM cells produced IL-1alpha constitutively, and the level of expression was increased after treatment with phacoemulsification ultrasound. As was previously demonstrated, the stress-regulated transcription factor NF-kappaB was present in the cytoplasm of normal cells, but in the nucleus of glaucomatous cells. After treatment with ultrasound energy, NF-kappaB translocated to the nucleus in the normal cells. Endothelial leukocyte-adhesion molecule (ELAM)-1 was not detected in normal TM cells, but was constitutively present on glaucomatous TM cells, consistent with findings in previous work. ELAM-1 expression was induced in normal cells by ultrasound treatment.
Conclusions: A potentially IOP-lowering stress response is induced in TM cells by ultrasound. The findings suggest that this response may be induced clinically during cataract removal by phacoemulsification, and may be one mechanism responsible for the reduction in IOP that often follows this procedure.
Figures



References
-
- Krohn J. Expression of factor VIII-related antigen in human aqueous drainage channels. Acta Ophthalmol Scand. 1999;77:9–12. - PubMed
-
- Foets B, van den Oord J, Engelmann K, Missotten L. A comparative immunohistochemical study of human corneotrabecular tissue. Graefes Arch Clin Exp Ophthalmol. 1992;230:269–274. - PubMed
-
- Kee C, Seo K. The effect of interleukin-1α on outflow facility in rat eyes. J Glaucoma. 1997;6:246–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

