Alpha-synuclein overexpression protects against paraquat-induced neurodegeneration
- PMID: 12716914
- PMCID: PMC6742319
- DOI: 10.1523/JNEUROSCI.23-08-03095.2003
Alpha-synuclein overexpression protects against paraquat-induced neurodegeneration
Abstract
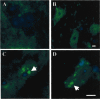
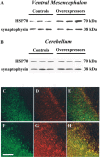
Alpha-synuclein is likely to play a role in neurodegenerative processes, including the degeneration of nigrostriatal dopaminergic neurons that underlies Parkinson's disease. However, the toxicological properties of alpha-synuclein remain relatively unknown. Here, the relationship between alpha-synuclein expression and neuronal injury was studied in mice exposed to the herbicide paraquat. Paraquat neurotoxicity was compared in control animals versus mice with transgenic expression of human alpha-synuclein driven by the tyrosine hydroxylase (TH) promoter. In control mice, paraquat caused both the formation of alpha-synuclein-containing intraneuronal deposits and the degeneration of nigrostriatal neurons, as demonstrated by silver staining and a reduction of the counts of TH-positive and Nissl-stained cells. Mice overexpressing alpha-synuclein, either the human wild-type or the Ala53Thr mutant form of the protein, displayed paraquat-induced protein aggregates but were completely protected against neurodegeneration. These resistant animals were also characterized by increased levels of HSP70, a chaperone protein that has been shown to counteract paraquat toxicity in other experimental models and could therefore contribute to neuroprotection in alpha-synuclein transgenic mice. The results indicate a dissociation between toxicant-induced alpha-synuclein deposition and neurodegeneration. They support a role of alpha-synuclein against toxic insults and suggest that its involvement in human neurodegenerative processes may arise not only from a gain of toxic function, as previously proposed, but also from a loss of defensive properties.
Figures

References
-
- Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of α-synuclein toxicity in a Drosophila model for Parkinson's disease. Science. 2002;295:865–868. - PubMed
-
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. - PubMed
-
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. - PubMed
-
- Clayton DF, George JM. The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998;21:249–254. - PubMed
-
- Cohen G. Enzymatic/nonenzymatic sources of oxyradicals and regulation of antioxidant defenses. Ann NY Acad Sci. 1994;738:8–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
