Independent sources of quantal variability at single glutamatergic synapses
- PMID: 12716926
- PMCID: PMC2944019
- DOI: 10.1523/JNEUROSCI.23-08-03186.2003
Independent sources of quantal variability at single glutamatergic synapses
Abstract
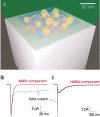
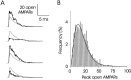
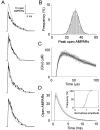
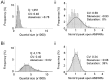
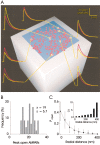
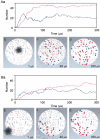
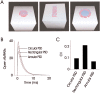
Variability in the size of single postsynaptic responses is a feature of most central neurons, although the source of this variability is not completely understood. The dominant source of variability could be either intersynaptic or intrasynaptic. To quantitatively examine this question, a biophysically realistic model of an idealized central axospinous synapse was used to assess mechanisms underlying synaptic variability measurements. Three independent sources of variability were considered: stochasticity of postsynaptic receptors ("channel noise"), variations of glutamate concentration in the synaptic cleft (Deltaq), and differences in the potency of vesicles released from different locations on the active zone [release-location dependence (RLD)]. As expected, channel noise was small (8% of the total variance) and Deltaq was the dominant source of variability (58% of total variance). Surprisingly, RLD accounted for a significant amount of variability (36%). Our simulations show that potency of release sites decreased with a length constant of approximately 100 nm, and that receptors were not activated by release events >300 nm away, which is consistent with the observation that single active zones are rarely >300 nm. RLD also predicts that the manner in which receptors are added or removed from synapses can dramatically affect the nature of the synaptic response, with increasing receptor density being more efficient than merely increasing synaptic area. Saturation levels and synaptic geometry were also important in determining the size and shape of the distribution of amplitudes recorded at different synapses.
Figures

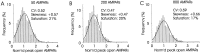
References
-
- Auger C, Marty A. Heterogeneity of functional synaptic parameters among single release sites. Neuron. 1997;19:139–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
