Activity-dependent trafficking and dynamic localization of zipcode binding protein 1 and beta-actin mRNA in dendrites and spines of hippocampal neurons
- PMID: 12716932
- PMCID: PMC6742306
- DOI: 10.1523/JNEUROSCI.23-08-03251.2003
Activity-dependent trafficking and dynamic localization of zipcode binding protein 1 and beta-actin mRNA in dendrites and spines of hippocampal neurons
Abstract
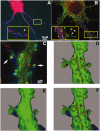
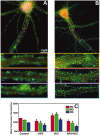
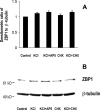
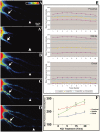
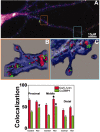
RNA binding proteins may be important mediators of the activity-dependent transport of mRNAs to dendritic spines of activated synapses. We used fluorescence microscopy and digital imaging techniques applied to both fixed and live cultured hippocampal neurons to visualize the localization of the mRNA binding protein, zipcode binding protein 1 (ZBP1), and its dynamic movements in response to KCl-induced depolarization at high spatial and temporal resolution. With the use of immunofluorescence, image deconvolution, and three-dimensional reconstruction, ZBP1 was localized in the form of granules that were distributed in dendrites, spines, and subsynaptic sites. KCl depolarization increased the dendritic localization of ZBP1 that was not attributed to an increase in ZBP1 expression. Live cell imaging of single cells before and after perfusion of KCl revealed the rapid and directed efflux of ZBP1 granules from the cell body into dendrites in a proximo-distal gradient. High-speed imaging of enhanced green fluorescence protein-ZBP1 granules revealed rapid anterograde and retrograde movements in dendrites as well as dynamic movements in dendritic spines. A population of ZBP1 granules colocalized with beta-actin mRNA, and their spatial association in dendrites was increased by KCl depolarization. The NMDA receptor antagonist AP-5 impaired the dendritic localization of ZBP1 and beta-actin mRNA and inhibited the KCl-induced transport of ZBP1. The activity-dependent trafficking of ZBP1 and its dynamic movements within dendritic spines provide new evidence to implicate RNA binding proteins as regulators of mRNA transport to activated synapses in response to synaptic activity.
Figures


References
-
- Bassell GJ, Singer RH. Neuronal mRNA localization and the cytoskeleton. In: Richter D, editor. Cell polarity and subcellular RNA localization. Springer; Berlin: 2001. pp. 41–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
