Heterogeneous nuclear ribonucleoprotein (hnRNP) binding to hormone response elements: a cause of vitamin D resistance
- PMID: 12716975
- PMCID: PMC156334
- DOI: 10.1073/pnas.1031395100
Heterogeneous nuclear ribonucleoprotein (hnRNP) binding to hormone response elements: a cause of vitamin D resistance
Abstract
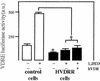
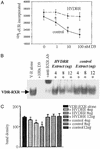
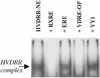
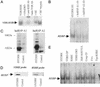
In previous studies, we have shown that steroid hormone resistance in New World primates occurs in the absence of abnormal expression of cognate nuclear receptors. Rather, these animals have elevated levels of heterogeneous nuclear ribonucleoproteins (hnRNPs) that act as hormone response element-binding proteins and attenuate target gene transactivation. Here we present evidence for a similar mechanism in humans via a patient with resistance to the active form of vitamin D [1,25-dihydroxyvitamin D(3) (1,25(OH)(2)D(3))] who presented with normal vitamin D receptor (VDR) expression. Initial cotransfection studies showed that the cells of the patient suppressed basal and hormone-induced transactivation by wild-type VDR. Electrophoretic mobility-shift assays and Western/Southwestern blot analyses indicated that this suppressive effect was due to overexpression of a nuclear protein that specifically interacts with a DNA response element known to bind retinoid X receptor-VDR heterodimers. Ab blocking in electrophoretic mobility-shift assays indicated that this dominant-negative acting protein was in the hnRNPA family of nucleic acid-binding proteins. Further studies have shown that several members of this family, most notably hnRNPA1, were able to suppress basal and 1,25(OH)(2)D(3)-induced luciferase activity. We therefore propose that this case of vitamin D resistance in a human subject is similar to that previously described for New World primates in which abnormal expression of a hormone response element-binding protein can cause target cell resistance to 1,25(OH)(2)D(3). That this protein is a member of the hnRNP family capable of interacting with double-stranded DNA highlights a potentially important new component of the complex machinery required for steroid hormone signal transduction.
Figures
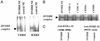
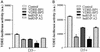
References
-
- Chrousos G P, MacLusky N J, Brandon D D, Tomita M, Renquist D M, Loriaux D L, Lipsett M B. Adv Exp Med Biol. 1986;196:317–328. - PubMed
-
- Auchus R J, Fuqua S A. Semin Cell Biol. 1994;5:127–136. - PubMed
-
- Ing N H, O'Malley B W. In: Molecular Endocrinology: Basic Concepts and Clinical Correlations. Weintraub B D, editor. New York: Raven; 1995. pp. 195–215.
-
- Chen J D, Li H. Crit Rev Eukaryotic Gene Expression. 1998;8:169–190. - PubMed
-
- Freedman L P. Cell. 1999;97:5–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

