Thrombin inhibits NMDA-mediated nociceptive activity in the mouse: possible mediation by endothelin
- PMID: 12717003
- PMCID: PMC2342990
- DOI: 10.1113/jphysiol.2002.036384
Thrombin inhibits NMDA-mediated nociceptive activity in the mouse: possible mediation by endothelin
Abstract
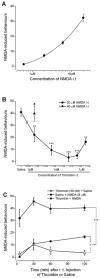
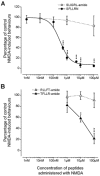
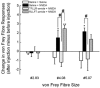
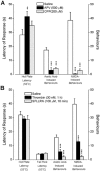
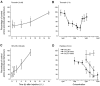
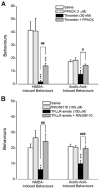
The CNS expresses many components of an extracellular protease signalling system, including the protease-activated receptor-1 (PAR-1) whose tethered ligand is generated by thrombin. Activation of PAR-1 potentiates NMDA receptor activity in hippocampal neurons. Because NMDA activity mediates hyperalgesia, we tested the hypothesis that PAR-1 receptors also regulate pain processing. In contrast to the potentiating effect of thrombin in the hippocampus, NMDA-induced behaviours and the transient mechanical hyperalgesia (von Frey fibres) induced by intrathecally injected NMDA in mice were inhibited by thrombin in a dose-related fashion. This anti-hyperalgesic effect was mimicked by SFLLRN, the natural ligand at PAR-1 binding sites, but not SLIGRL-amide, a PAR-2 agonist. The effects of SFLLRN were less potent and shorter in duration than that of thrombin, consistent with its more transient effect on PAR-1 sites. Both thrombin and SFLLRN inhibited acetic acid-induced abdominal stretch (writhing) behaviours, which were also sensitive to NMDA antagonism, but not hot plate or tail flick latencies, which were insensitive to NMDA antagonists. TFLLR-amide, a selective ligand for PAR-1 sites, mimicked the effects of thrombin while RLLFT-amide, an inactive, reverse peptide sequence, did not. In addition, the effect of TFLLR-amide was prevented by RWJ-56110, a PAR-1 antagonist. Thrombin and TFLLR-amide produced no oedema (Evans Blue extravasation) in the spinal cord that would account for these effects. Based on the reported ability of thrombin to mobilize endothelin-1 from astrocytes, we tested the role of this compound in thrombin's activity. BQ123, an endothelin A receptor antagonist, prevented thrombin's inhibition of writhing and NMDA-induced behaviours while BQ788, an endothelin B receptor antagonist, did not. Thus, activation of PAR-1 sites by thrombin in the CNS appears to inhibit NMDA-mediated nociception by a pathway involving endothelin type A receptors.
Figures

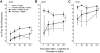

References
-
- Aanonsen LM, Wilcox GL. Phencyclidine selectively blocks a spinal action of N-methyl-d-aspartate in mice. Neurosci Lett. 1986;67:191–197. - PubMed
-
- Andrade-Gordon P, Maryanoff BE, Derian CK, Zhang HC, Addo MF, Darrow AL, Eckardt AJ, Hoekstra WJ, McComsey DF, Oksenberg D, Reynolds EE, Santulli RJ, Scarborough RM, Smith CE, White KB. Design, synthesis, and biological characterization of a peptide-mimetic antagonist for a tethered-ligand receptor. Proc Natl Acad Sci U S A. 1999;96:12 257–12 262. - PMC - PubMed
-
- Bjorkman R. Central antinociceptive effects of non-steroidal anti-inflammatory drugs and paracetamol. Experimental studies in the rat. Acta Anesthesiol Scand. 1995;103(suppl.):1–44. - PubMed
-
- Boyce S, Wyatt A, Webb JK, O'Donnell R, Mason G, Rigby M, Sirinathsinghji D, Hill RG, Rupniak NM. Selective NMDA NR2B antagonists induce antinociception without motor dysfunction: correlation with restricted localisation of NR2B subunit in dorsal horn. Neuropharmacology. 1999;38:611–623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

