Energy-dependent degradation: Linkage between ClpX-catalyzed nucleotide hydrolysis and protein-substrate processing
- PMID: 12717012
- PMCID: PMC2323860
- DOI: 10.1110/ps.0237603
Energy-dependent degradation: Linkage between ClpX-catalyzed nucleotide hydrolysis and protein-substrate processing
Abstract
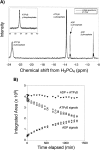
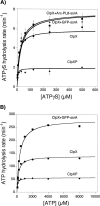
ClpX requires ATP to unfold protein substrates and translocate them into the proteolytic chamber of ClpP for degradation. The steady-state parameters for hydrolysis of ATP and ATPgammaS by ClpX were measured with different protein partners and the kinetics of degradation of ssrA-tagged substrates were determined with both nucleotides. ClpX hydrolyzed ATPgammaS to ADP and thiophosphate at a rate (6/min) significantly slower than ATP hydrolysis (140/min), but the hydrolysis of both nucleotides was increased by ssrA-tagged substrates and decreased by ClpP. K(M) and k(cat) for hydrolysis of ATP and ATPgammaS were linearly correlated over a 200-fold range, suggesting that protein partners largely affect k(cat) rather than nucleotide binding, indicating that most bound ATP leaves the enzyme by hydrolysis rather than dissociation, and placing an upper limit of approximately 15 micro M on K(D) for both nucleotides. Competition studies with ClpX and fluorescently labeled ADP gave inhibition constants for ATPgammaS ( approximately 2 micro M) and ADP ( approximately 3 micro M) under the reaction conditions used for steady-state kinetics. In the absence of Mg(2+), where hydrolysis does not occur, the inhibition constant for ATP ( approximately 55 micro M) was weaker but very similar to the value for ATPgammaS ( approximately 45 micro M). Compared with ATP, ATPgammaS supported slow but roughly comparable rates of ClpXP degradation for two Arc-ssrA substrates and denatured GFP-ssrA, but not of native GFP-ssrA. These results show that the processing of protein substrates by ClpX is closely coupled to the maximum rate of nucleotide hydrolysis.
Figures




References
-
- Burton, B.M., Williams, T.L., and Baker, T.A. 2001. ClpX-mediated remodeling of Mu transpososomes: Selective unfolding of subunits destabilizes the entire complex. Mol. Cell 8 449–454. - PubMed
-
- Conaway, R.C., Brower, C.S., and Conaway, J.W. 2002. Emerging roles of ubiquitin in transcription regulation. Science 296 1254–1258. - PubMed
-
- Eckstein, F. and Goody, R.S. 1976. Synthesis and properties of diastereomers of adenosine 5′-(O-1-thiotriphosphate) and adenosine 5′-(O-2-thiotriphosphate). Biochemistry 15 1685–1691. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

