Arresting and releasing Staphylococcal alpha-hemolysin at intermediate stages of pore formation by engineered disulfide bonds
- PMID: 12717022
- PMCID: PMC2323870
- DOI: 10.1110/ps.0231203
Arresting and releasing Staphylococcal alpha-hemolysin at intermediate stages of pore formation by engineered disulfide bonds
Abstract
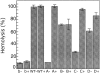
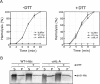
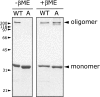
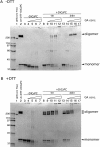
alpha-Hemolysin (alphaHL) is secreted by Staphylococcus aureus as a water-soluble monomer that assembles into a heptamer to form a transmembrane pore on a target membrane. The crystal structures of the LukF water-soluble monomer and the membrane-bound alpha-hemolysin heptamer show that large conformational changes occur during assembly. However, the mechanism of assembly and pore formation is still unclear, primarily because of the difficulty in obtaining structural information on assembly intermediates. Our goal is to use disulfide bonds to selectively arrest and release alphaHL from intermediate stages of the assembly process and to use these mutants to test mechanistic hypotheses. To accomplish this, we created four double cysteine mutants, D108C/K154C (alphaHL-A), M113C/K147C (alphaHL-B), H48C/ N121C (alphaHL-C), I5C/G130C (alphaHL-D), in which disulfide bonds may form between the pre-stem domain and the beta-sandwich domain to prevent pre-stem rearrangement and membrane insertion. Among the four mutants, alphaHL-A is remarkably stable, is produced at a level at least 10-fold greater than that of the wild-type protein, is monomeric in aqueous solution, and has hemolytic activity that can be regulated by the presence or absence of reducing agents. Cross-linking analysis showed that alphaHL-A assembles on a membrane into an oligomer, which is likely to be a heptamer, in the absence of a reducing agent, suggesting that oxidized alphaHL-A is halted at a heptameric prepore state. Therefore, conformational rearrangements at positions 108 and 154 are critical for the completion of alphaHL assembly but are not essential for membrane binding or for formation of an oligomeric prepore intermediate.
Figures



Similar articles
-
Controlling pore assembly of staphylococcal gamma-haemolysin by low temperature and by disulphide bond formation in double-cysteine LukF mutants.Mol Microbiol. 2002 Sep;45(6):1485-98. doi: 10.1046/j.1365-2958.2002.03125.x. Mol Microbiol. 2002. PMID: 12354220
-
Heptameric structures of two alpha-hemolysin mutants imaged with in situ atomic force microscopy.Microsc Res Tech. 1999 Mar 1;44(5):353-6. doi: 10.1002/(SICI)1097-0029(19990301)44:5<353::AID-JEMT6>3.0.CO;2-0. Microsc Res Tech. 1999. PMID: 10090210
-
Role of the amino latch of staphylococcal alpha-hemolysin in pore formation: a co-operative interaction between the N terminus and position 217.J Biol Chem. 2006 Jan 27;281(4):2195-204. doi: 10.1074/jbc.M510841200. Epub 2005 Oct 14. J Biol Chem. 2006. PMID: 16227199
-
Beta-barrel membrane protein folding and structure viewed through the lens of alpha-hemolysin.Biochim Biophys Acta. 2003 Jan 10;1609(1):19-27. doi: 10.1016/s0005-2736(02)00663-6. Biochim Biophys Acta. 2003. PMID: 12507754 Review.
-
alpha-Hemolysin from Staphylococcus aureus: an archetype of beta-barrel, channel-forming toxins.J Struct Biol. 1998;121(2):110-22. doi: 10.1006/jsbi.1998.3959. J Struct Biol. 1998. PMID: 9615434 Review.
Cited by
-
Auto-Assembling Detoxified Staphylococcus aureus Alpha-Hemolysin Mimicking the Wild-Type Cytolytic Toxin.Clin Vaccine Immunol. 2016 Jun 6;23(6):442-50. doi: 10.1128/CVI.00091-16. Print 2016 Jun. Clin Vaccine Immunol. 2016. PMID: 27030589 Free PMC article.
-
Anti-alpha-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia.Infect Immun. 2009 Jul;77(7):2712-8. doi: 10.1128/IAI.00115-09. Epub 2009 Apr 20. Infect Immun. 2009. PMID: 19380475 Free PMC article.
-
Structure-based discovery of a small-molecule inhibitor of methicillin-resistant Staphylococcus aureus virulence.J Biol Chem. 2020 May 1;295(18):5944-5959. doi: 10.1074/jbc.RA120.012697. Epub 2020 Mar 16. J Biol Chem. 2020. PMID: 32179646 Free PMC article.
-
Trapping a 96 degrees domain rotation in two distinct conformations by engineered disulfide bridges.Protein Sci. 2004 Jul;13(7):1811-22. doi: 10.1110/ps.04629604. Protein Sci. 2004. PMID: 15215524 Free PMC article.
-
Identification of a key residue for oligomerisation and pore-formation of Clostridium perfringens NetB.Toxins (Basel). 2014 Mar 12;6(3):1049-61. doi: 10.3390/toxins6031049. Toxins (Basel). 2014. PMID: 24625763 Free PMC article.
References
-
- Booth, P.J., Templer, R.H., Meijberg, W., Allen, S.J., Curran, A.R., and Lorch, M. 2001. In vitro studies of membrane protein folding. Crit. Rev. Biochem. Mol. Biol. 36 501–603. - PubMed
-
- Czajkowsky, D.M., Sheng, S., and Shao, Z. 1998. Staphylococcal α-hemolysin can form hexamers in phospholipid bilayers. J. Mol. Biol. 276 325–330. - PubMed
-
- Falke, J.J. and Koshland, D.E. 1987. Global flexibility in a sensory receptor: A site-directed cross-linking approach. Science 237 1596–1600. - PubMed
-
- Fang, Y., Cheley, S., Bayley, H., and Yang, J. 1997. The heptameric prepore of a Staphylococcal α-hemolysin mutant in lipid bilayers imaged by atomic force microscopy. Biochemistry 36 9518–9522. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

