Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum
- PMID: 12717445
- PMCID: PMC3572851
- DOI: 10.1038/ncb980
Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum
Abstract
Calcium is a second messenger in virtually all cells and tissues. Calcium signals in the nucleus have effects on gene transcription and cell growth that are distinct from those of cytosolic calcium signals; however, it is unknown how nuclear calcium signals are regulated. Here we identify a reticular network of nuclear calcium stores that is continuous with the endoplasmic reticulum and the nuclear envelope. This network expresses inositol 1,4,5-trisphosphate (InsP3) receptors, and the nuclear component of InsP3-mediated calcium signals begins in its locality. Stimulation of these receptors with a little InsP3 results in small calcium signals that are initiated in this region of the nucleus. Localized release of calcium in the nucleus causes nuclear protein kinase C (PKC) to translocate to the region of the nuclear envelope, whereas release of calcium in the cytosol induces translocation of cytosolic PKC to the plasma membrane. Our findings show that the nucleus contains a nucleoplasmic reticulum with the capacity to regulate calcium signals in localized subnuclear regions. The presence of such machinery provides a potential mechanism by which calcium can simultaneously regulate many independent processes in the nucleus.
Figures


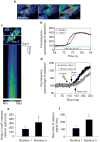

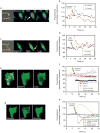
Comment in
-
Calcium is good for the nucleus.J Hepatol. 2004 Apr;40(4):720-2. doi: 10.1016/j.jhep.2004.02.012. J Hepatol. 2004. PMID: 15030997 No abstract available.
References
-
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nature Rev Mol Cell Biol. 2000;1:11–21. - PubMed
-
- Jouaville LS, Ichas F, Holmuhamedov EL, Camacho P, Lechleiter JD. Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature. 1995;377:438–441. - PubMed
-
- Stehno-Bittel L, Perez-Terzic C, Clapham DE. Diffusion across the nuclear envelope inhibited by depletion of the nuclear Ca2+ store. Science. 1995;270:1835–1838. - PubMed
-
- Perez-Terzic C, Pyle J, Jaconi M, Stehno-Bittel L, Clapham DE. Conformational states of the nuclear pore complex induced by depletion of nuclear Ca2+ stores. Science. 1996;273:1875–1877. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

