Noninvasive imaging of lentiviral-mediated reporter gene expression in living mice
- PMID: 12718911
- PMCID: PMC4155985
- DOI: 10.1016/s1525-0016(03)00070-4
Noninvasive imaging of lentiviral-mediated reporter gene expression in living mice
Abstract
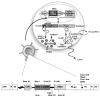
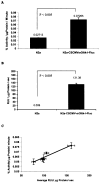
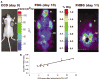
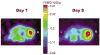
Lentiviral-mediated gene delivery holds significant promise for sustained gene expression within living systems. Vesicular stomatitis virus glycoprotein-pseudotyped human immunodeficiency virus type 1-based lentiviral vectors can be used to introduce transgenes in a broad spectrum of dividing as well as nondividing cells. In the current study, we construct a lentiviral vector carrying two reporter genes separated by an internal ribosomal entry site and utilize that virus in delivering both genes into neuroblastoma cells in cell culture and into cells implanted in living mice. We utilize two reporter genes, a mutant herpes simplex virus type 1 (HSV1) sr39tk as a reporter gene compatible with positron emission tomography (PET) and a bioluminescent optical reporter gene, firefly luciferase (Fluc), to image expression in living mice by an optical charge-coupled device (CCD) camera. By using this lentivirus, neuroblastoma (N2a) cells are stably transfected and a high correlation (R(2) = 0.91) between expressions of the two reporter genes in cell culture is established. Imaging of both reporter genes using microPET and optical CCD camera in living mice is feasible, with the optical approach being more sensitive, and a high correlation (R(2) = 0.86) between gene expressions is again observed in lentiviral-infected N2a tumor xenografts. Indirect imaging of HSV1-sr39tk suicide gene therapy utilizing Fluc is also feasible and can be detected with increased sensitivity by using the optical CCD. These preliminary results validate the use of lentiviral vectors carrying reporter genes for multimodality imaging of gene expression and should have many applications, including imaging of xenografts, metastasis, and cell trafficking as well as noninvasive monitoring of lentiviral-mediated gene delivery and expression.
Figures


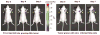
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

