Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2
- PMID: 12719479
- PMCID: PMC2193968
- DOI: 10.1084/jem.20021787
Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2
Abstract
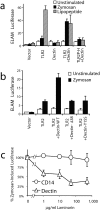
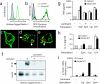
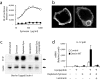
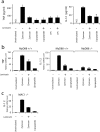
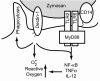
Toll-like receptors (TLRs) mediate recognition of a wide range of microbial products including lipopolysaccharides, lipoproteins, flagellin, and bacterial DNA, and signaling through TLRs leads to the production of inflammatory mediators. In addition to TLRs, many other surface receptors have been proposed to participate in innate immunity and microbial recognition, and signaling through some of these receptors is likely to cooperate with TLR signaling in defining inflammatory responses. In this report we have examined how dectin-1, a lectin family receptor for beta-glucans, collaborates with TLRs in recognizing microbes. Dectin-1, which is expressed at low levels on macrophages and high levels on dendritic cells, contains an immunoreceptor tyrosine-based activation motif-like signaling motif that is tyrosine phosphorylated upon activation. The receptor is recruited to phagosomes containing zymosan particles but not to phagosomes containing immunoglobulin G-opsonized particles. Dectin-1 expression enhances TLR-mediated activation of nuclear factor kappa B by beta-glucan-containing particles, and in macrophages and dendritic cells dectin-1 and TLRs are synergistic in mediating production of cytokines such as interleukin 12 and tumor necrosis factor alpha. Additionally, dectin-1 triggers production of reactive oxygen species, an inflammatory response that is primed by TLR activation. The data demonstrate that collaborative recognition of distinct microbial components by different classes of innate immune receptors is crucial in orchestrating inflammatory responses.
Figures


References
-
- Underhill, D.M., and A. Ozinsky. 2002. Phagocytosis of microbes: complexity in action. Annu. Rev. Immunol. 20:825–852. - PubMed
-
- Henson, P.M., D.L. Bratton, and V.A. Fadok. 2001. Apoptotic cell removal. Curr. Biol. 11:R795–R805. - PubMed
-
- Underhill, D.M., and A. Ozinsky. 2002. Toll-like receptors: key mediators of microbe detection. Curr. Opin. Immunol. 14:103–110. - PubMed
-
- Aderem, A., and R.J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature. 406:782–787. - PubMed
-
- Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675–680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

