The voltage-sensitive release mechanism of excitation contraction coupling in rabbit cardiac muscle is explained by calcium-induced calcium release
- PMID: 12719483
- PMCID: PMC2217377
- DOI: 10.1085/jgp.200208764
The voltage-sensitive release mechanism of excitation contraction coupling in rabbit cardiac muscle is explained by calcium-induced calcium release
Abstract
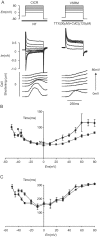
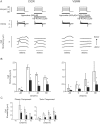
The putative voltage-sensitive release mechanism (VSRM) was investigated in rabbit cardiac myocytes at 37 degrees C with high resistance microelectrodes to minimize intracellular dialysis. When the holding potential was adjusted from -40 to -60 mV, the putative VSRM was expected to operate alongside CICR. Under these conditions however, we did not observe a plateau at positive potentials of the cell shortening versus voltage relationship. The threshold for cell shortening changed by -10 mV, but this resulted from a similar change of the threshold for activation of inward current. Cell shortening under conditions where the putative VSRM was expected to operate was blocked in a dose dependent way by nifedipine and CdCl2 and blocked completely by NiCl2. "Tail contractions" persisted in the presence of nifedipine and CdCl2 but were blocked completely by NiCl2. Block of early outward current by 4-aminopyridine and 4-acetoamido-4'-isothiocyanato-stilbene-2,2'-disulfonic acid (SITS) demonstrated persisting inward current during test depolarizations despite the presence of nifedipine and CdCl2. Inward current did not persist in the presence of NiCl2. A tonic component of cell shortening that was prominent during depolarizations to positive potentials under conditions selective for the putative VSRM was sensitive to rapidly applied changes in superfusate [Na+] and to the outward Na+/Ca2+ exchange current blocking drug KB-R7943. This component of cell shortening was thought to be the result of Na+/Ca2+ exchange-mediated excitation contraction coupling. Cell shortening recorded under conditions selective for the putative VSRM was increased by the enhanced state of phosphorylation induced by isoprenaline (1 microM) and by enhancing sarcoplasmic reticulum Ca2+ content by manipulation of the conditioning steps. Under these conditions, cell shortening at positive test depolarizations was converted from tonic to phasic. We conclude that the putative VSRM is explained by CICR with the Ca2+ "trigger" supplied by unblocked L-type Ca2+ channels and Na+/Ca2+ exchange.
Figures











Comment in
-
Excitation contraction coupling in cardiac muscle: is there a purely voltage-dependent component?J Gen Physiol. 2003 May;121(5):349-52. doi: 10.1085/jgp.200308841. J Gen Physiol. 2003. PMID: 12719482 Free PMC article. No abstract available.
Similar articles
-
In contrast to forskolin and 3-isobutyl-1-methylxanthine, amrinone stimulates the cardiac voltage-sensitive release mechanism without increasing calcium-induced calcium release.J Pharmacol Exp Ther. 2001 Sep;298(3):954-63. J Pharmacol Exp Ther. 2001. PMID: 11504790
-
Dihydropyridine receptors functioning as voltage sensors in cardiac myocytes.J Physiol Pharmacol. 2000 Dec;51(4 Pt 2):777-98. J Physiol Pharmacol. 2000. PMID: 11220488
-
Voltage dependence of cardiac excitation-contraction coupling: unitary Ca2+ current amplitude and open channel probability.Circ Res. 2007 Sep 14;101(6):590-7. doi: 10.1161/CIRCRESAHA.107.152322. Epub 2007 Jul 19. Circ Res. 2007. PMID: 17641229
-
[Regulation of calcium liberation in sarcoplasmic reticulum and heart muscle cells].Verh K Acad Geneeskd Belg. 1997;59(5):401-34. Verh K Acad Geneeskd Belg. 1997. PMID: 9490926 Review. Dutch.
-
Inactivation of L-type calcium channels in cardiomyocytes. Experimental and theoretical approaches.Gen Physiol Biophys. 2003 Dec;22(4):441-54. Gen Physiol Biophys. 2003. PMID: 15113117 Review.
Cited by
-
A novel quantitative explanation for the autonomic modulation of cardiac pacemaker cell automaticity via a dynamic system of sarcolemmal and intracellular proteins.Am J Physiol Heart Circ Physiol. 2010 Jun;298(6):H2010-23. doi: 10.1152/ajpheart.00783.2009. Epub 2010 Mar 12. Am J Physiol Heart Circ Physiol. 2010. PMID: 20228256 Free PMC article.
-
Mechanisms of transition from normal to reentrant electrical activity in a model of rabbit atrial tissue: interaction of tissue heterogeneity and anisotropy.Biophys J. 2009 Feb;96(3):798-817. doi: 10.1016/j.bpj.2008.09.057. Biophys J. 2009. PMID: 19186122 Free PMC article.
-
Effects of ORAI calcium release-activated calcium modulator 1 (ORAI1) on neutrophil activity in dairy cows with subclinical hypocalcemia1.J Anim Sci. 2019 Jul 30;97(8):3326-3336. doi: 10.1093/jas/skz209. J Anim Sci. 2019. PMID: 31299068 Free PMC article.
-
Excitation contraction coupling in cardiac muscle: is there a purely voltage-dependent component?J Gen Physiol. 2003 May;121(5):349-52. doi: 10.1085/jgp.200308841. J Gen Physiol. 2003. PMID: 12719482 Free PMC article. No abstract available.
References
-
- Allen, T.J.A., and R.A. Chapman. 1992. Temperature dependence of L-type calcium currents in single isolated guinea-pig ventricular myocytes. J. Physiol. 446:554P.
-
- Barcenas Ruiz, L., D.J. Beuckelmann, and W.G. Wier. 1987. Sodium-calcium exchange in heart: membrane currents and changes in [Ca2+]i. Science. 238:1720–1722. - PubMed
-
- Barcenas Ruiz, L., and W.G. Wier. 1987. Voltage dependence of intracellular [Ca2+]i transients in guinea pig ventricular myocytes. Circ. Res. 61:148–154. - PubMed
-
- Bassani, J.W., W. Yuan, and D.M. Bers. 1995. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am. J. Physiol. 268:C1313–C1319. - PubMed
-
- Berlin, J.R., M.B. Cannell, and W.J. Lederer. 1987. Regulation of twitch tension in sheep cardiac Purkinje fibers during calcium overload. Am. J. Physiol. 253:H1540–H1547. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

