Hydrophobic coupling of lipid bilayer energetics to channel function
- PMID: 12719487
- PMCID: PMC2217378
- DOI: 10.1085/jgp.200308797
Hydrophobic coupling of lipid bilayer energetics to channel function
Abstract
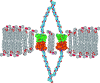
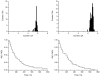
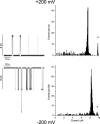
The hydrophobic coupling between membrane-spanning proteins and the lipid bilayer core causes the bilayer thickness to vary locally as proteins and other "defects" are embedded in the bilayer. These bilayer deformations incur an energetic cost that, in principle, could couple membrane proteins to each other, causing them to associate in the plane of the membrane and thereby coupling them functionally. We demonstrate the existence of such bilayer-mediated coupling at the single-molecule level using single-barreled as well as double-barreled gramicidin channels in which two gramicidin subunits are covalently linked by a water-soluble, flexible linker. When a covalently attached pair of gramicidin subunits associates with a second attached pair to form a double-barreled channel, the lifetime of both channels in the assembly increases from hundreds of milliseconds to a hundred seconds--and the conductance of each channel in the side-by-side pair is almost 10% higher than the conductance of the corresponding single-barreled channels. The double-barreled channels are stabilized some 100,000-fold relative to their single-barreled counterparts. This stabilization arises from: first, the local increase in monomer concentration around a single-barreled channel formed by two covalently linked gramicidins, which increases the rate of double-barreled channel formation; and second, from the increased lifetime of the double-barreled channels. The latter result suggests that the two barrels of the construct associate laterally. The underlying cause for this lateral association most likely is the bilayer deformation energy associated with channel formation. More generally, the results suggest that the mechanical properties of the host bilayer may cause the kinetics of membrane protein conformational transitions to depend on the conformational states of the neighboring proteins.
Figures


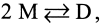
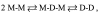

Similar articles
-
Single-molecule methods for monitoring changes in bilayer elastic properties.Methods Mol Biol. 2007;400:543-70. doi: 10.1007/978-1-59745-519-0_37. Methods Mol Biol. 2007. PMID: 17951759 Review.
-
The conformational preference of gramicidin channels is a function of lipid bilayer thickness.FEBS Lett. 1997 Jul 21;412(1):15-20. doi: 10.1016/s0014-5793(97)00709-6. FEBS Lett. 1997. PMID: 9257681
-
Genistein can modulate channel function by a phosphorylation-independent mechanism: importance of hydrophobic mismatch and bilayer mechanics.Biochemistry. 2003 Nov 25;42(46):13646-58. doi: 10.1021/bi034887y. Biochemistry. 2003. PMID: 14622011
-
Spring constants for channel-induced lipid bilayer deformations. Estimates using gramicidin channels.Biophys J. 1999 Feb;76(2):889-95. doi: 10.1016/S0006-3495(99)77252-8. Biophys J. 1999. PMID: 9929490 Free PMC article.
-
Bilayer thickness and membrane protein function: an energetic perspective.Annu Rev Biophys Biomol Struct. 2007;36:107-30. doi: 10.1146/annurev.biophys.36.040306.132643. Annu Rev Biophys Biomol Struct. 2007. PMID: 17263662 Review.
Cited by
-
Structure-based prediction of drug distribution across the headgroup and core strata of a phospholipid bilayer using surrogate phases.Mol Pharm. 2014 Oct 6;11(10):3577-95. doi: 10.1021/mp5003366. Epub 2014 Sep 18. Mol Pharm. 2014. PMID: 25179490 Free PMC article.
-
Ligand-dependent conformations and dynamics of the serotonin 5-HT(2A) receptor determine its activation and membrane-driven oligomerization properties.PLoS Comput Biol. 2012;8(4):e1002473. doi: 10.1371/journal.pcbi.1002473. Epub 2012 Apr 19. PLoS Comput Biol. 2012. PMID: 22532793 Free PMC article.
-
Regulation of Antimicrobial Peptide Activity via Tuning Deformation Fields by Membrane-Deforming Inclusions.Int J Mol Sci. 2021 Dec 28;23(1):326. doi: 10.3390/ijms23010326. Int J Mol Sci. 2021. PMID: 35008752 Free PMC article.
-
Bilayer thickness modulates the conductance of the BK channel in model membranes.Biophys J. 2004 Jun;86(6):3620-33. doi: 10.1529/biophysj.103.029678. Biophys J. 2004. PMID: 15189859 Free PMC article.
-
Gating gramicidin channels in lipid bilayers: reaction coordinates and the mechanism of dissociation.Biophys J. 2004 Jan;86(1 Pt 1):92-104. doi: 10.1016/S0006-3495(04)74087-4. Biophys J. 2004. PMID: 14695253 Free PMC article.
References
-
- Alberts, B., A. Johnson, J. Lewis, M. Raff, K. Roberts, and P. Walter. 2002. Molecular Biology of the Cell. 4th Edition. Garland Science, New York.
-
- Almers, W., and C. Stirling. 1984. Distribution of transport proteins over animal cell membranes. J. Membr. Biol. 77:169–186. - PubMed
-
- Andersen, O.S. 1978. Ion transport across simple membranes. Renal Function. G.H. Giebisch and E.F. Purcell, editors. The Josiah Macy, Jr. Foundation. 71–99.

