Functional conservation of light, oxygen, or voltage domains in light sensing
- PMID: 12719523
- PMCID: PMC156305
- DOI: 10.1073/pnas.1031791100
Functional conservation of light, oxygen, or voltage domains in light sensing
Abstract
In Neurospora, the flavin adenine dinucleotide-containing protein WHITE COLLAR-1 is the blue-light photoreceptor for the circadian clock and other light responses. The putative chromophore-binding domain of WC-1, its light, oxygen, or voltage (LOV) domain, is similar to the LOV domains found in the plant phototropins, the Neurospora VIVID (VVD) protein, and the Arabidopsis FKF1 and its related proteins. Studies of the plant phototropins have identified 11 flavin-contacting residues that are also conserved in the LOV domains of WC-1, VVD, and FKF1. In this study, by mutating the putative WC-1 flavin-binding sites, we show that these sites are important for the light function of the protein, suggesting that the WC-1 LOV domain adapts a structure similar to that of the phototropin LOV domains. By creating a Neurospora strain in which the LOV domain of WC-1 is swapped with that of VVD, we show that the LOV domain of VVD partially replaces the function of the WC-1 LOV domain, suggesting that VVD is a wc-dependent photoreceptor in Neurospora. Furthermore, we show that the Neurosporastrains containing a chimeric WC-1 protein with the LOV domain from FKF1 or phot1 can also sense light, suggesting that FKF1 and its related proteins are light sensors in Arabidopsis. Taken together, our data suggest that these LOV domains are structurally similar protein modules involved in blue-light sensing.
Figures

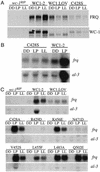
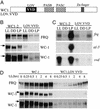

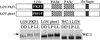
References
-
- Briggs W R, Huala E. Annu Rev Cell Dev Biol. 1999;15:33–62. - PubMed
-
- Devlin P F, Kay S A. Annu Rev Physiol. 2001;63:677–694. - PubMed
-
- Sancar A. Annu Rev Biochem. 2000;69:31–67. - PubMed
-
- Linden H, Ballario P, Macino G. Fungal Genet Biol. 1997;22:141–150. - PubMed
-
- Cashmore A R, Jarillo J A, Wu Y J, Liu D. Science. 1999;284:760–765. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

