Evolutionary conservation predicts function of variants of the human organic cation transporter, OCT1
- PMID: 12719534
- PMCID: PMC156299
- DOI: 10.1073/pnas.0730858100
Evolutionary conservation predicts function of variants of the human organic cation transporter, OCT1
Abstract
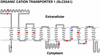
The organic cation transporter, OCT1, is a major hepatic transporter that mediates the uptake of many organic cations from the blood into the liver where the compounds may be metabolized or secreted into the bile. Because OCT1 interacts with a variety of structurally diverse organic cations, including clinically used drugs as well as toxic substances (e.g., N-methylpyridinium, MPP(+)), it is an important determinant of systemic exposure to many xenobiotics. To understand the genetic basis of extensive interindividual differences in xenobiotic disposition, we functionally characterized 15 protein-altering variants of the human liver organic cation transporter, OCT1, in Xenopus oocytes. All variants that reduced or eliminated function (OCT1-R61C, OCT1-P341L, OCT1-G220V, OCT1-G401S, and OCT1-G465R) altered evolutionarily conserved amino acid residues. In general, variants with decreased function had amino acid substitutions that resulted in more radical chemical changes (higher Grantham values) and were less evolutionarily favorable (lower blosum62 values) than variants that maintained function. A variant with increased function (OCT1-S14F) changed an amino acid residue such that the human protein matched the consensus of the OCT1 mammalian orthologs. Our results indicate that changes at evolutionarily conserved positions of OCT1 are strong predictors of decreased function and suggest that a combination of evolutionary conservation and chemical change might be a stronger predictor of function.
Figures



References
-
- Evans W E, Relling M V. Science. 1999;286:487–491. - PubMed
-
- Zhang L, Schaner M E, Giacomini K M. J Pharmacol Exp Ther. 1998;286:354–361. - PubMed
-
- Zhang L, Brett C M, Giacomini K M. Annu Rev Pharmacol Toxicol. 1998;38:431–460. - PubMed
-
- Zhang L, Dresser M J, Gray A T, Yost S C, Terashita S, Giacomini K M. Mol Pharmacol. 1997;51:913–921. - PubMed
-
- Gorboulev V, Ulzheimer J C, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S, Baumann C, Lang F, Busch A E, Koepsell H. DNA Cell Biol. 1997;16:871–881. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

