Mammalian linker-histone subtypes differentially affect gene expression in vivo
- PMID: 12719535
- PMCID: PMC156302
- DOI: 10.1073/pnas.0736105100
Mammalian linker-histone subtypes differentially affect gene expression in vivo
Abstract
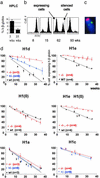
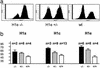
Posttranslational modifications and remodeling of nucleosomes are critical factors in the regulation of transcription. Higher-order folding of chromatin also is likely to contribute to the control of gene expression, but the absence of a detailed description of the structure of the chromatin fiber has impaired progress in this area. Mammalian somatic cells contain a set of H1 linker-histone subtypes, H1 (0) and H1a to H1e, that bind to nucleosome core particles and to the linker DNA between nucleosomes. To determine whether the H1 histone subtypes play differential roles in the regulation of gene expression, we combined mice lacking specific H1 histone subtypes with mice carrying transgenes subject to position effects. Because position effects result from the unique chromatin structure created by the juxtaposition of regulatory elements in the transgene and at the site of integration, transgenes can serve as exquisitely sensitive indicators of chromatin structure. We report that some, but not all, linker histones can attenuate or accentuate position effects. The results suggest that the linker-histone subtypes play differential roles in the control of gene expression and that the sequential arrangement of the linker histones on the chromatin fiber might regulate higher-order chromatin structure and fine-tune expression levels.
Figures


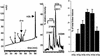
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

