Parkin, a gene implicated in autosomal recessive juvenile parkinsonism, is a candidate tumor suppressor gene on chromosome 6q25-q27
- PMID: 12719539
- PMCID: PMC156308
- DOI: 10.1073/pnas.0931262100
Parkin, a gene implicated in autosomal recessive juvenile parkinsonism, is a candidate tumor suppressor gene on chromosome 6q25-q27
Expression of concern in
-
Editorial Expression of Concern: Parkin, a gene implicated in autosomal recessive juvenile parkinsonism, is a candidate tumor suppressor gene on chromosome 6q25-q27.Proc Natl Acad Sci U S A. 2017 Apr 18;114(16):E3364. doi: 10.1073/pnas.1704295114. Epub 2017 Apr 3. Proc Natl Acad Sci U S A. 2017. PMID: 28373549 Free PMC article. No abstract available.
Abstract
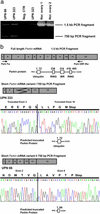
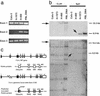
In an effort to identify tumor suppressor gene(s) associated with the frequent loss of heterozygosity observed on chromosome 6q25-q27, we constructed a contig derived from the sequences of bacterial artificial chromosomeP1 bacteriophage artificial chromosome clones defined by the genetic interval D6S1581-D6S1579-D6S305-D6S1599-D6S1008. Sequence analysis of this contig found it to contain eight known genes, including the complete genomic structure of the Parkin gene. Loss of heterozygosity (LOH) analysis of 40 malignant breast and ovarian tumors identified a common minimal region of loss, including the markers D6S305 (50%) and D6S1599 (32%). Both loci exhibited the highest frequencies of LOH in this study and are each located within the Parkin genomic structure. Whereas mutation analysis revealed no missense substitutions, expression of the Parkin gene appeared to be down-regulated or absent in the tumor biopsies and tumor cell lines examined. In addition, the identification of two truncating deletions in 3 of 20 ovarian tumor samples, as well as homozygous deletion of exon 2 in the lung adenocarcinoma cell lines Calu-3 and H-1573, supports the hypothesis that hemizygous or homozygous deletions are responsible for the abnormal expression of Parkin in these samples. These data suggest that the LOH observed at chromosome 6q25-q26 may contribute to the initiation andor progression of cancer by inactivating or reducing the expression of the Parkin gene. Because Parkin maps to FRA6E, one of the most active common fragile sites in the human genome, it represents another example of a large tumor suppressor gene, like FHIT and WWOX, located at a common fragile site.
Figures
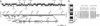

References
-
- Hanahan D, Weinberg R A. Cell. 2000;100:57–70. - PubMed
-
- Hinds P W, Weinberg R A. Curr Opin Genet Dev. 1994;4:135–141. - PubMed
-
- Lin J C, Scherer S W, Tougas L, Traverso G, Tsui L C, Andrulis I L, Jothy S, Park M. Oncogene. 1996;13:2001–2008. - PubMed
-
- Saito S, Sirahama S, Matsushima M, Suzuki M, Sagae S, Kudo R, Saito J, Noda K, Nakamura Y. Cancer Res. 1996;56:5586–5589. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

