L-particle production during primary replication of pseudorabies virus in the nasal mucosa of swine
- PMID: 12719558
- PMCID: PMC154012
- DOI: 10.1128/jvi.77.10.5657-5667.2003
L-particle production during primary replication of pseudorabies virus in the nasal mucosa of swine
Abstract
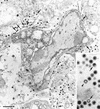
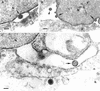
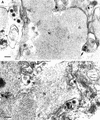
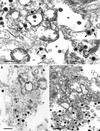
Different tissue culture cell lines infected with a number of alphaherpesviruses produce, in addition to virions, light particles (L particles). L particles are composed of the envelope and tegument components of the virion but totally lack the proteins of the capsid and the virus genome; therefore, they are noninfectious. In this electron microscopy report, we show that L particles are produced during primary replication of the alphaherpesvirus pseudorabies virus (PRV) in the nasal mucosa of experimentally infected swine, its natural host. Although PRV infected different types of cells of the respiratory and olfactory mucosae, PRV L particles were found to be produced exclusively by epithelial cells and fibroblasts. We observed that formation of noninfectious particles occurred by budding of condensed tegument at the inner nuclear membrane and at membranes of cytoplasmic vesicles, resulting in intracisternal and intravesicular L particles, respectively. Both forms of capsidless particles were clearly distinguishable by the presence of prominent surface projections on the envelope and the higher electron density of the tegument, morphological features which were only observed in intravesicular L particles. Moreover, intravesicular but not intracisternal L particles were found to be released by exocytosis and were also identified extracellularly. Comparative analysis between PRV virion and L-particle morphogenesis indicates that both types of virus particles share a common intracellular pathway of assembly and egress but that they show different production patterns during the replication cycle of PRV.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

