Positive regulation of CXCR4 expression and signaling by interleukin-7 in CD4+ mature thymocytes correlates with their capacity to favor human immunodeficiency X4 virus replication
- PMID: 12719571
- PMCID: PMC154045
- DOI: 10.1128/jvi.77.10.5784-5793.2003
Positive regulation of CXCR4 expression and signaling by interleukin-7 in CD4+ mature thymocytes correlates with their capacity to favor human immunodeficiency X4 virus replication
Abstract
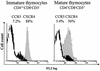
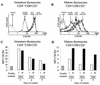
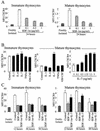
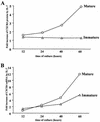
The emergence of X4 human immunodeficiency virus type 1 (HIV-1) variants in infected individuals is associated with poor prognosis. One of the possible causes of this emergence might be the selection of X4 variants in some specific tissue compartment. We demonstrate that the thymic microenvironment favors the replication of X4 variants by positively modulating the expression and signaling of CXCR4 in mature CD4(+) CD8(-) CD3(+) thymocytes. Here, we show that the interaction of thymic epithelial cells (TEC) with these thymocytes in culture induces an upregulation of CXCR4 expression. The cytokine secreted by TEC, interleukin-7 (IL-7), increases cell surface expression of CXCR4 and efficiently overcomes the downregulation induced by SDF-1 alpha, also produced by TEC. IL-7 also potentiates CXCR4 signaling, leading to actin polymerization, a process necessary for virus entry. In contrast, in intermediate CD4(+) CD8(-) CD3(-) thymocytes, the other subpopulation known to allow virus replication, TEC or IL-7 has little or no effect on CXCR4 expression and signaling. CCR5 is expressed at similarly low levels in the two thymocyte subpopulations, and neither its expression nor its signaling was modified by the cytokines tested. This positive regulation of CXCR4 by IL-7 in mature CD4(+) thymocytes correlates with their high capacity to favor X4 virus replication compared with intermediate thymocytes or peripheral blood mononuclear cells. Indeed, we observed an enrichment of X4 viruses after replication in thymocytes initially infected with a mixture of X4 (NL4-3) and R5 (NLAD8) HIV strains and after the emergence of X4 variants from an R5 primary isolate during culture in mature thymocytes.
Figures
Similar articles
-
Thymocyte-thymic epithelial cell interaction leads to high-level replication of human immunodeficiency virus exclusively in mature CD4(+) CD8(-) CD3(+) thymocytes: a critical role for tumor necrosis factor and interleukin-7.J Virol. 1999 Sep;73(9):7533-42. doi: 10.1128/JVI.73.9.7533-7542.1999. J Virol. 1999. PMID: 10438843 Free PMC article.
-
Inhibition of CD3/CD28-mediated activation of the MEK/ERK signaling pathway represses replication of X4 but not R5 human immunodeficiency virus type 1 in peripheral blood CD4(+) T lymphocytes.J Virol. 2000 Mar;74(6):2558-66. doi: 10.1128/jvi.74.6.2558-2566.2000. J Virol. 2000. PMID: 10684270 Free PMC article.
-
Envelope-dependent restriction of human immunodeficiency virus type 1 spreading in CD4(+) T lymphocytes: R5 but not X4 viruses replicate in the absence of T-cell receptor restimulation.J Virol. 1999 Sep;73(9):7515-23. doi: 10.1128/JVI.73.9.7515-7523.1999. J Virol. 1999. PMID: 10438841 Free PMC article.
-
Cytokines in the thymus: production and biological effects.Curr Med Chem. 2004 Feb;11(4):447-64. doi: 10.2174/0929867043455972. Curr Med Chem. 2004. PMID: 14965226 Review.
-
HIV-1 replication in CD4+ T cell lines: the effects of adaptation on co-receptor use, tropism, and accessory gene function.J Leukoc Biol. 2000 Sep;68(3):331-7. J Leukoc Biol. 2000. PMID: 10985248 Review.
Cited by
-
R5 human immunodeficiency virus type 1 infection of fetal thymic organ culture induces cytokine and CCR5 expression.J Virol. 2005 Jan;79(1):458-71. doi: 10.1128/JVI.79.1.458-471.2005. J Virol. 2005. PMID: 15596839 Free PMC article.
-
Altered responses to homeostatic cytokines in patients with idiopathic CD4 lymphocytopenia.PLoS One. 2013;8(1):e55570. doi: 10.1371/journal.pone.0055570. Epub 2013 Jan 30. PLoS One. 2013. PMID: 23383227 Free PMC article.
-
Transforming growth factor-beta1 upregulates the expression of CXC chemokine receptor 4 (CXCR4) in human breast cancer MCF-7 cells.Acta Pharmacol Sin. 2010 Mar;31(3):347-54. doi: 10.1038/aps.2009.204. Epub 2010 Feb 15. Acta Pharmacol Sin. 2010. PMID: 20154716 Free PMC article.
-
HIV Impacts CD34+ Progenitors Involved in T-Cell Differentiation During Coculture With Mouse Stromal OP9-DL1 Cells.Front Immunol. 2019 Jan 29;10:81. doi: 10.3389/fimmu.2019.00081. eCollection 2019. Front Immunol. 2019. PMID: 30761146 Free PMC article.
-
Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment.J Clin Invest. 2009 Apr;119(4):997-1007. doi: 10.1172/JCI38052. Epub 2009 Mar 16. J Clin Invest. 2009. PMID: 19287090 Free PMC article. Clinical Trial.
References
-
- Aldrovandi, G. M., G. Feuer, L. Gao, B. Jamieson, M. Kristeva, I. S. Chen, and J. A. Zack. 1993. The SCID-hu mouse as a model for HIV-1 infection. Nature 363:732-736. - PubMed
-
- Amara, A., S. L. Gall, O. Schwartz, J. Salamero, M. Montes, P. Loetscher, M. Baggiolini, J. L. Virelizier, and F. Arenzana-Seisdedos. 1997. HIV coreceptor downregulation as antiviral principle: SDF-1α-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J. Exp. Med. 186:139-146. - PMC - PubMed
-
- Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. - PubMed
-
- Berkowitz, R. D., K. P. Beckerman, T. J. Schall, and J. M. McCune. 1998. CXCR4 and CCR5 expression delineates targets for HIV-1 disruption of T cell differentiation. J. Immunol. 161:3702-3710. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

