Human immunodeficiency virus type 1 Env with an intersubunit disulfide bond engages coreceptors but requires bond reduction after engagement to induce fusion
- PMID: 12719576
- PMCID: PMC154041
- DOI: 10.1128/jvi.77.10.5829-5836.2003
Human immunodeficiency virus type 1 Env with an intersubunit disulfide bond engages coreceptors but requires bond reduction after engagement to induce fusion
Abstract
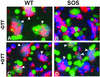
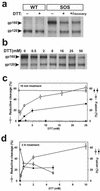
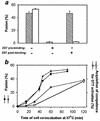
A mutant human immunodeficiency virus (HIV) envelope protein (Env) with an engineered disulfide bond between the gp120 and gp41 subunits (SOS-Env) was expressed on cell surfaces. With the disulfide bond intact, these cells did not fuse to target cells expressing CD4 and CCR5, but the fusion process did advance to an intermediate state: cleaving the disulfide bond with a reducing agent after but not before binding to target cells allowed fusion to occur. Through the use of an antibody directed against CCR5, it was found that at the intermediate stage, SOS-Env had associated with coreceptors. Reducing the disulfide bond after this intermediate had been reached resulted in hemifusion at low temperature and fusion at physiological temperature. The addition of C34 or N36, peptides that prevent six-helix bundle formation, at the hemifused state blocked the fusion that would have resulted after raising the temperature. Thus, Env has not yet folded into six-helix bundles after hemifusion has been achieved. Because SOS-Env binds CCR5, it is suggested that the conformational changes in wild-type Env that result from this binding cause disengagement of gp120 from gp41 in the region of the engineered bond. It is proposed that this disengagement is the event that directly frees gp41 to undergo the conformational changes that lead to fusion. The intermediate state achieved prior to reduction of the disulfide bond was stable. The capture of this configuration of Env could yield a suitable antigen for vaccine development, and it may also be a target for pharmacological intervention against HIV-1 entry.
Figures
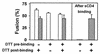


References
-
- Allan, J. S., J. E. Coligan, F. Barin, M. F. McLane, J. G. Sodroski, C. A. Rosen, W. A. Haseltine, T. H. Lee, and M. Essex. 1985. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science 228:1091-1094. - PubMed
-
- Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. - PubMed
-
- Binley, J. M., R. W. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. J. Anselma, P. J. Maddon, W. C. Olson, and J. P. Moore. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74:627-643. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

