Differentiation of varicella-zoster virus ORF47 protein kinase and IE62 protein binding domains and their contributions to replication in human skin xenografts in the SCID-hu mouse
- PMID: 12719588
- PMCID: PMC154036
- DOI: 10.1128/jvi.77.10.5964-5974.2003
Differentiation of varicella-zoster virus ORF47 protein kinase and IE62 protein binding domains and their contributions to replication in human skin xenografts in the SCID-hu mouse
Abstract
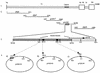
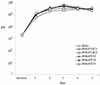
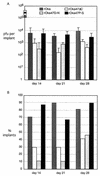
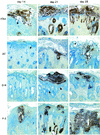
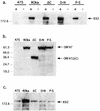
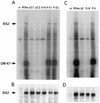
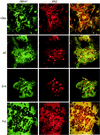
To investigate the role of the ORF47 protein kinase of varicella-zoster virus (VZV), we constructed VZV recombinants with targeted mutations in conserved motifs of ORF47 and a truncated ORF47 and characterized these mutants for replication, phosphorylation, and protein-protein interactions in vitro and for infectivity in human skin xenografts in the SCID-hu mouse model in vivo. Previous experiments showed that ROka47S, a null mutant that makes no ORF47 protein, did not replicate in skin in vivo (J. F. Moffat, L. Zerboni, M. H. Sommer, T. C. Heineman, J. I. Cohen, H. Kaneshima, and A. M. Arvin, Proc. Natl. Acad. Sci. USA 95:11969-11974, 1998). The construction of VZV recombinants with targeted ORF47 mutations made it possible to assess the effects on VZV infection of human skin xenografts of selectively abolishing ORF47 protein kinase activity. ORF47 mutations that resulted in a C-terminal truncation or disrupted the DYS kinase motif eliminated ORF47 kinase activity and were associated with extensive nuclear retention of ORF47 and IE62 proteins in vitro. Disrupting ORF47 kinase function also resulted in a marked decrease in VZV replication and cutaneous lesion formation in skin xenografts in vivo. However, infectivity in vivo was not blocked completely as long as the capacity of ORF47 protein to bind IE62 protein was preserved, a function that we identified and mapped to the N-terminal domain of ORF47 protein. These experiments indicate that ORF47 kinase activity is of critical importance for VZV infection and cell-cell spread in human skin in vivo but suggest that it is the formation of complexes between ORF47 and IE62 proteins, both VZV tegument components, that constitutes the essential contribution of ORF47 protein to VZV replication in vivo.
Figures

References
-
- Arvin, A. M. 2001. Varicella-zoster virus, p. 2731-2767. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott, Philadelphia, Pa.
-
- Cohen, J. I., and S. E. Straus. 2001. Varicella-zoster virus and its replication, p. 2707-2730. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott, Philadelphia, Pa.
-
- Grose, C. 1981. Variation on a theme by Fenner: the pathogenesis of chickenpox. Pediatrics 68:735-737. - PubMed
-
- Hanks, S. K., and T. Hunter. 1995. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9:576-596. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

