Threonine 157 of influenza virus PA polymerase subunit modulates RNA replication in infectious viruses
- PMID: 12719592
- PMCID: PMC154019
- DOI: 10.1128/jvi.77.10.6007-6013.2003
Threonine 157 of influenza virus PA polymerase subunit modulates RNA replication in infectious viruses
Abstract
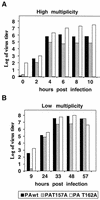
Previous results have shown a correlation between the decrease in protease activity of several influenza A virus PA protein mutants and the capacity to replicate of the corresponding mutant ribonucleoproteins (RNPs) reconstituted in vivo. In this work we studied the phenotype of mutant viruses containing these mutations. Viruses with a T162A mutation, which showed a very moderate decrease both in protease and replication activities of reconstituted RNPs, showed a wild-type phenotype. Viruses with a T157A mutation, which presented a severe decrease in protease activity and replication of RNPs, showed a complex phenotype: (i) transport to the nucleus of PAT157A protein was delayed, (ii) virus multiplication was reduced at both low and high multiplicities, (iii) transcriptive synthesis was unaltered while replicative synthesis, especially cRNA, was diminished, and (iv) viral pathogenesis in mice was reduced, as measured by loss of body weight and virus titers in lungs. Finally, recombinant viruses with a T157E mutation in PA protein, which resulted in a drastic reduction of protease and replication activities of RNPs, were not viable. These results indicate that residue T157 in PA protein is important for the capacity of viral polymerase to synthesize cRNA.
Figures

Similar articles
-
Comparative analysis of the ability of the polymerase complexes of influenza viruses type A, B and C to assemble into functional RNPs that allow expression and replication of heterotypic model RNA templates in vivo.Virology. 1999 Dec 20;265(2):342-53. doi: 10.1006/viro.1999.0059. Virology. 1999. PMID: 10600605
-
A single amino acid mutation in the PA subunit of the influenza virus RNA polymerase inhibits endonucleolytic cleavage of capped RNAs.J Virol. 2002 Sep;76(18):8989-9001. doi: 10.1128/jvi.76.18.8989-9001.2002. J Virol. 2002. PMID: 12186883 Free PMC article.
-
Mutations in the N-terminal region of influenza virus PB2 protein affect virus RNA replication but not transcription.J Virol. 2003 May;77(9):5098-108. doi: 10.1128/jvi.77.9.5098-5108.2003. J Virol. 2003. PMID: 12692212 Free PMC article.
-
The RNA polymerase of influenza a virus: mechanisms of viral transcription and replication.Acta Virol. 2013;57(2):113-22. doi: 10.4149/av_2013_02_113. Acta Virol. 2013. PMID: 23600869 Review.
-
Structure and Function of Influenza Virus Ribonucleoprotein.Subcell Biochem. 2018;88:95-128. doi: 10.1007/978-981-10-8456-0_5. Subcell Biochem. 2018. PMID: 29900494 Review.
Cited by
-
The contribution of PA-X to the virulence of pandemic 2009 H1N1 and highly pathogenic H5N1 avian influenza viruses.Sci Rep. 2015 Feb 5;5:8262. doi: 10.1038/srep08262. Sci Rep. 2015. PMID: 25652161 Free PMC article.
-
Genetic and pathogenic characterisation of 11 avian reovirus isolates from northern China suggests continued evolution of virulence.Sci Rep. 2016 Oct 18;6:35271. doi: 10.1038/srep35271. Sci Rep. 2016. PMID: 27752067 Free PMC article.
-
Defective assembly of influenza A virus due to a mutation in the polymerase subunit PA.J Virol. 2006 Jan;80(1):252-61. doi: 10.1128/JVI.80.1.252-261.2006. J Virol. 2006. PMID: 16352550 Free PMC article.
-
Amino acid residues in the N-terminal region of the PA subunit of influenza A virus RNA polymerase play a critical role in protein stability, endonuclease activity, cap binding, and virion RNA promoter binding.J Virol. 2006 Aug;80(16):7789-98. doi: 10.1128/JVI.00600-06. J Virol. 2006. PMID: 16873236 Free PMC article.
-
Mutational analyses of the influenza A virus polymerase subunit PA reveal distinct functions related and unrelated to RNA polymerase activity.PLoS One. 2012;7(1):e29485. doi: 10.1371/journal.pone.0029485. Epub 2012 Jan 6. PLoS One. 2012. PMID: 22238617 Free PMC article.
References
-
- Almond, J. W., and V. Felsenreich. 1982. Phosphorylation of the nucleoprotein of an avian influenza virus. J. Gen. Virol. 60:295-305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

