Science review: role of coagulation protease cascades in sepsis
- PMID: 12720558
- PMCID: PMC270604
- DOI: 10.1186/cc1825
Science review: role of coagulation protease cascades in sepsis
Abstract
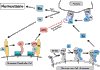
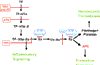
Cellular signaling by proteases of the blood coagulation cascade through members of the protease-activated receptor (PAR) family can profoundly impact on the inflammatory balance in sepsis. The coagulation initiation reaction on tissue factor expressing cells signals through PAR1 and PAR2, leading to enhanced inflammation. The anticoagulant protein C pathway has potent anti-inflammatory effects, and activated protein C signals through PAR1 upon binding to the endothelial protein C receptor. Activation of the coagulation cascade and the downstream endothelial cell localized anticoagulant pathway thus have opposing effects on systemic inflammation. This dichotomy is of relevance for the interpretation of preclinical and clinical data that document nonuniform responses to anticoagulant strategies in sepsis therapy.
Figures

Comment in
-
Coagulation cascade in sepsis: getting from bench to bedside?Crit Care. 2003 Apr;7(2):117-8. doi: 10.1186/cc1842. Epub 2002 Nov 6. Crit Care. 2003. PMID: 12720555 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

