Folding quality control in the export of proteins by the bacterial twin-arginine translocation pathway
- PMID: 12721369
- PMCID: PMC156335
- DOI: 10.1073/pnas.0937838100
Folding quality control in the export of proteins by the bacterial twin-arginine translocation pathway
Abstract
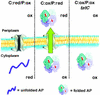
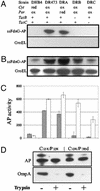
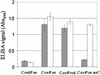
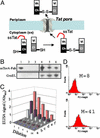
To examine the relationship between folding and export competence by the twin-arginine translocation (Tat) pathway we analyzed the subcellular localization of fusions between a set of eight putative Tat leader peptides and alkaline phosphatase in isogenic Escherichia coli strains that either allow or disfavor the formation of protein disulfide bonds in the cytoplasm. We show that export by the Tat translocator is observed only in strains that enable oxidative protein folding in the cytoplasm. Further, we show that other disulfide-containing proteins, namely single-chain Fv and heterodimeric F(AB) antibody fragments, are export-competent only in strains having an oxidizing cytoplasm. Functional, heterodimeric F(AB) protein was exported from the cytoplasm by means of a Tat leader peptide fused to the heavy chain alone, indicating that the formation of a disulfide-bonded dimer preceeds export. These results demonstrate that in vivo only proteins that have attained the native conformation are exported by the Tat translocator, indicating that a folding quality-control mechanism is intrinsic to the export process. The ability to export proteins with disulfide bonds and the folding proofing feature of the Tat pathway are of interest for biotechnology applications.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

