Resistance of cell membranes to different detergents
- PMID: 12721375
- PMCID: PMC156280
- DOI: 10.1073/pnas.0631579100
Resistance of cell membranes to different detergents
Abstract
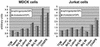
Partial resistance of cell membranes to solubilization with mild detergents and the analysis of isolated detergent-resistant membranes (DRMs) have been used operationally to define membrane domains. Given the multitude of detergents used for this purpose, we sought to investigate whether extraction with different detergents might reflect the same underlying principle of domain formation. We therefore compared the protein and lipid content of DRMs prepared with a variety of detergents from two cell lines. We found that the detergents differ considerably in their ability to selectively solubilize membrane proteins and to enrich sphingolipids and cholesterol over glycerophospholipids as well as saturated over unsaturated phosphatidylcholine. In addition, we observed cell type-dependent variations of the molecular characteristics of DRMs and the effectiveness of particular detergents. These results make it unlikely that different detergents reflect the same aspects of membrane organization and underscore both the structural complexity of cell membranes and the need for more sophisticated analytical tools to understand their architecture.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

