Mossy fiber plasticity and enhanced hippocampal excitability, without hippocampal cell loss or altered neurogenesis, in an animal model of prolonged febrile seizures
- PMID: 12722980
- PMCID: PMC2927853
- DOI: 10.1002/hipo.10089
Mossy fiber plasticity and enhanced hippocampal excitability, without hippocampal cell loss or altered neurogenesis, in an animal model of prolonged febrile seizures
Abstract
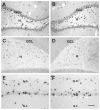
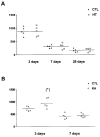
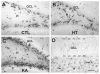
Seizures induced by fever (febrile seizures) are the most frequent seizures affecting infants and children; however, their impact on the developing hippocampal formation is not completely understood. Such understanding is highly important because of the potential relationship of prolonged febrile seizures to temporal lobe epilepsy. Using an immature rat model, we have previously demonstrated that prolonged experimental febrile seizures render the hippocampus hyperexcitable throughout life. Here we examined whether (1) neuronal loss, (2) altered neurogenesis, or (3) mossy fiber sprouting, all implicated in epileptogenesis in both animal models and humans, were involved in the generation of a pro-epileptic, hyperexcitable hippocampus by these seizures. The results demonstrated that prolonged experimental febrile seizures did not result in appreciable loss of any vulnerable hippocampal cell population, though causing strikingly enhanced sensitivity to hippocampal excitants later in life. In addition, experimental febrile seizures on postnatal day 10 did not enhance proliferation of granule cells, whereas seizures generated by kainic acid during the same developmental age increased neurogenesis in the immature hippocampus. However, prolonged febrile seizures resulted in long-term axonal reorganization in the immature hippocampal formation: Mossy fiber densities in granule cell- and molecular layers were significantly increased by 3 months (but not 10 days) after the seizures. Thus, the data indicate that prolonged febrile seizures influence connectivity of the immature hippocampus long-term, and this process requires neither significant neuronal loss nor altered neurogenesis. In addition, the temporal course of the augmented mossy fiber invasion of the granule cell and molecular layers suggests that it is a consequence, rather than the cause, of the hyperexcitable hippocampal network resulting from these seizures.
Figures




References
-
- Adams B, Lee M, Fahnestock M, Racine RJ. Long-term potentiation trains induce mossy fiber sprouting. Brain Res. 1997;775:193–197. - PubMed
-
- Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990;301:365–381. - PubMed
-
- André V, Marescaux C, Nehlig A, Fritschy JM. Alterations of hippocampal GABAergic system contribute to development of spontaneous recurrent seizures in the lithium-pilocarpine model of temporal lobe epilepsy. Hippocampus. 2001;11:452–468. - PubMed
-
- Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF. Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience. 1991;42:351–363. - PubMed

