Osteoclast-independent bone resorption by fibroblast-like cells
- PMID: 12723988
- PMCID: PMC165048
- DOI: 10.1186/ar752
Osteoclast-independent bone resorption by fibroblast-like cells
Abstract
To date, mesenchymal cells have only been associated with bone resorption indirectly, and it has been hypothesized that the degradation of bone is associated exclusively with specific functions of osteoclasts. Here we show, in aseptic prosthesis loosening, that aggressive fibroblasts at the bone surface actively contribute to bone resorption and that this is independent of osteoclasts. In two separate models (a severe combined immunodeficient mouse coimplantation model and a dentin pit formation assay), these cells produce signs of bone resorption that are similar to those in early osteoclastic resorption. In an animal model of aseptic prosthesis loosening (i.e. intracranially self-stimulated rats), it is shown that these fibroblasts acquire their ability to degrade bone early on in their differentiation. Upon stimulation, such fibroblasts readily release acidic components that lower the pH of their pericellular milieu. Through the use of specific inhibitors, pericellular acidification is shown to involve the action of vacuolar type ATPases. Although fibroblasts, as mesenchymal derived cells, are thought to be incapable of resorbing bone, the present study provides the first evidence to challenge this widely held belief. It is demonstrated that fibroblast-like cells, under pathological conditions, may not only enhance but also actively contribute to bone resorption. These cells should therefore be considered novel therapeutic targets in the treatment of bone destructive disorders.
Figures

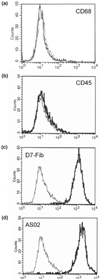

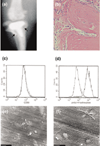

Similar articles
-
Activation of dimeric glucocorticoid receptors in osteoclast progenitors potentiates RANKL induced mature osteoclast bone resorbing activity.Bone. 2016 Dec;93:43-54. doi: 10.1016/j.bone.2016.08.024. Epub 2016 Sep 2. Bone. 2016. PMID: 27596806
-
Characterization of different osteoclast phenotypes in the progression of bone invasion by oral squamous cell carcinoma.Oncol Rep. 2018 Mar;39(3):1043-1051. doi: 10.3892/or.2017.6166. Epub 2017 Dec 19. Oncol Rep. 2018. PMID: 29286135 Free PMC article.
-
Disease status in autosomal dominant osteopetrosis type 2 is determined by osteoclastic properties.J Bone Miner Res. 2006 Jul;21(7):1089-97. doi: 10.1359/jbmr.060409. J Bone Miner Res. 2006. PMID: 16813529
-
The role of osteoclast differentiation in aseptic loosening.J Orthop Res. 2002 Jan;20(1):1-8. doi: 10.1016/S0736-0266(01)00070-5. J Orthop Res. 2002. PMID: 11855378 Review.
-
Osteoclast function and bone-resorbing activity: An overview.Biochem Biophys Res Commun. 2016 Jul 29;476(3):115-20. doi: 10.1016/j.bbrc.2016.05.019. Epub 2016 May 5. Biochem Biophys Res Commun. 2016. PMID: 27157135 Review.
Cited by
-
Fibroblast-like cells change gene expression of bone remodelling markers in transwell cultures.Eur J Med Res. 2020 Oct 29;25(1):52. doi: 10.1186/s40001-020-00453-y. Eur J Med Res. 2020. PMID: 33121539 Free PMC article.
-
Nano wear particles and the periprosthetic microenvironment in aseptic loosening induced osteolysis following joint arthroplasty.Front Cell Infect Microbiol. 2023 Oct 3;13:1275086. doi: 10.3389/fcimb.2023.1275086. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37854857 Free PMC article. Review.
-
Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair.Adv Drug Deliv Rev. 2015 Nov 1;94:53-62. doi: 10.1016/j.addr.2015.03.013. Epub 2015 Apr 8. Adv Drug Deliv Rev. 2015. PMID: 25861724 Free PMC article. Review.
-
Are fibroblasts involved in joint destruction?Ann Rheum Dis. 2005 Nov;64 Suppl 4(Suppl 4):iv52-4. doi: 10.1136/ard.2005.042424. Ann Rheum Dis. 2005. PMID: 16239388 Free PMC article. Review. No abstract available.
-
The role played by cell-substrate interactions in the pathogenesis of osteoclast-mediated peri-implant osteolysis.Arthritis Res Ther. 2006;8(3):R70. doi: 10.1186/ar1938. Epub 2006 Apr 13. Arthritis Res Ther. 2006. PMID: 16613614 Free PMC article.
References
-
- Goldring SR, Schiller AL, Roelke M, Rourke CM, O'Neil DA, Harris WH. The synovial-like membrane at the bone-cement interface in loose total hip replacements and its proposed role in bone lysis. J Bone Joint Surg [Am] 1983;65:575–584. - PubMed
-
- Lafyatis R, Remmers EF, Roberts AB, Yocum DE, Sporn MB, Wilder RL. Anchorage-independent growth of synoviocytes from arthritic and normal joints. Stimulation by exogenous platelet-derived growth factor and inhibition by transforming growth factor-beta and retinoids. J Clin Invest. 1989;83:1267–1276. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

