Receptor-coupled, DAG-gated Ca2+-permeable cationic channels in LNCaP human prostate cancer epithelial cells
- PMID: 12724346
- PMCID: PMC2342876
- DOI: 10.1113/jphysiol.2002.036772
Receptor-coupled, DAG-gated Ca2+-permeable cationic channels in LNCaP human prostate cancer epithelial cells
Abstract
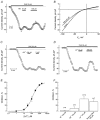
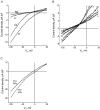
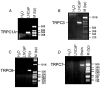
Although the prostate gland is a rich source of alpha1-adreno- (alpha1-AR) and m1-cholino receptors (m1-AChR), the membrane processes associated with their activation in glandular epithelial cells is poorly understood. We used the whole-cell patch-clamp technique to show that the agonists of the respective receptors, phenylephrine (PHE) and carbachol (CCh), activate cationic membrane currents in lymph node carcinoma of the prostate (LNCaP) human prostate cancer epithelial cells, which are not dependent on the filling status of intracellular IP3-sensitive Ca2+ stores, but directly gated by diacylglycerol (DAG), as evidenced by the ability of its membrane permeable analogue, OAG, to mimic the effects of the agonists. The underlying cationic channels are characterized by the weak field-strength Eisenman IV permeability sequence for monovalent cations (PK(25) > PCs(4.6) > PLi(1.4) > PNa(1.0)), and the following permeability sequence for divalent cations: PCa(1.0) > PMg(0.74) > PBa(0.6) > PSr(0.36) > PMn(0.3). They are 4.3 times more permeable to Ca2+ than Na+ and more sensitive to the inhibitor 2-APB than SK&F 96365. RT-PCR analysis shows that DAG-gated members of the transient receptor potential (TRP) channel family, including TRPC1 and TRPC3, are present in LNCaP cells. We conclude that, in prostate cancer epithelial cells, alpha1-ARs and m1-AChRs are functionally coupled to Ca2+-permeable DAG-gated cationic channels, for which TRPC1 and TRPC3 are the most likely candidates.
Figures




References
-
- Berridge MJ. Calcium signalling and cell proliferation. Bioessays. 1995;17:491–500. - PubMed
-
- Berridge MJ, Bootman MD, Lipp P. Calcium - a life and death signal. Nature. 1998;935:645–648. - PubMed
-
- Brough GH, Wu S, Cioffi D, Moore TM, Li M, Dean N, Stevens T. Contribution of endogenously expressed Trp1 to a Ca2+-selective, store-operated Ca2+ entry pathway. FASEB J. 2001;15:1727–1738. - PubMed
-
- Braun FJ, Broad LM, Armstrong DL, Putney JW., Jr Stable activation of single Ca2+ release-activated Ca2+ channels in divalent cation-free solutions. J Biol Chem. 2001;276:1063–1070. - PubMed
-
- Brauner-Osborne H, Brann MR. Pharmacology of muscarinic acetylcholine receptor subtypes (m1-m5): high throughput assays in mammalian cells. Eur J Pharmacol. 1996;295:93–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

