The mechanism of Mus81-Mms4 cleavage site selection distinguishes it from the homologous endonuclease Rad1-Rad10
- PMID: 12724407
- PMCID: PMC164751
- DOI: 10.1128/MCB.23.10.3487-3496.2003
The mechanism of Mus81-Mms4 cleavage site selection distinguishes it from the homologous endonuclease Rad1-Rad10
Abstract
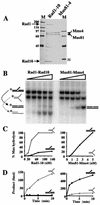
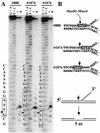
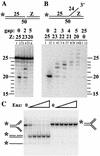
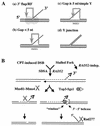
Mus81-Mms4 and Rad1-Rad10 are homologous structure-specific endonucleases that cleave 3' branches from distinct substrates and are required for replication fork stability and nucleotide excision repair, respectively, in the yeast Saccharomyces cerevisiae. We explored the basis of this biochemical and genetic specificity. The Mus81-Mms4 cleavage site, a nick 5 nucleotides (nt) 5' of the flap, is determined not by the branch point, like Rad1-Rad10, but by the 5' end of the DNA strand at the flap junction. As a result, the endonucleases show inverse substrate specificity; substrates lacking a 5' end within 4 nt of the flap are cleaved poorly by Mus81-Mms4 but are cleaved well by Rad1-10. Genetically, we show that both mus81 and sgs1 mutants are sensitive to camptothecin-induced DNA damage. Further, mus81 sgs1 synthetic lethality requires homologous recombination, as does suppression of mutant phenotypes by RusA expression. These data are most easily explained by a model in which the in vivo substrate of Mus81-Mms4 and Sgs1-Top3 is a 3' flap recombination intermediate downstream of replication fork collapse.
Figures



References
-
- Bardwell, A. J., L. Bardwell, A. E. Tomkinson, and E. C. Friedberg. 1994. Specific cleavage of model recombination and repair intermediates by the yeast Rad1-Rad10 DNA endonuclease. Science 265:2082-2085. - PubMed
-
- Bennett, R. J., M. F. Noirot-Gros, and J. C. Wang. 2000. Interaction between yeast Sgs1 helicase and DNA topoisomerase III. J. Biol. Chem. 275:26898-26905. - PubMed
-
- Boddy, M. N., P. H. Gaillard, W. H. McDonald, P. Shanahan, J. R. Yates III, and P. Russell. 2001. Mus81-Eme1 are essential components of a Holliday junction resolvase. Cell 107:537-548. - PubMed
-
- Bolt, E. L., and R. G. Lloyd. 2002. Substrate specificity of RusA resolvase reveals the DNA structures targeted by RuvAB and RecG in vivo. Mol. Cell 10:187-198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
