Constitutive E2F1 overexpression delays endochondral bone formation by inhibiting chondrocyte differentiation
- PMID: 12724423
- PMCID: PMC164752
- DOI: 10.1128/MCB.23.10.3656-3668.2003
Constitutive E2F1 overexpression delays endochondral bone formation by inhibiting chondrocyte differentiation
Abstract
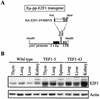
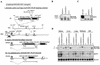
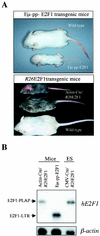
Longitudinal bone growth results from endochondral ossification, a process that requires proliferation and differentiation of chondrocytes. It has been shown that proper endochondral bone formation is critically dependent on the retinoblastoma family members p107 and p130. However, the precise functional roles played by individual E2F proteins remain poorly understood. Using both constitutive and conditional E2F1 transgenic mice, we show that ubiquitous transgene-driven expression of E2F1 during embryonic development results in a dwarf phenotype and significantly reduced postnatal viability. Overexpression of E2F1 disturbs chondrocyte maturation, resulting in delayed endochondral ossification, which is characterized by reduced hypertrophic zones and disorganized growth plates. Employing the chondrogenic cell line ATDC5, we investigated the effects of enforced E2F expression on the different phases of chondrocyte maturation that are normally required for endochondral ossification. Ectopic E2F1 expression strongly inhibits early- and late-phase differentiation of ATDC5 cells, accompanied by diminished cartilage nodule formation as well as decreased type II collagen, type X collagen, and aggrecan gene expression. In contrast, overexpression of E2F2 or E2F3a results in only a marginal delay of chondrocyte maturation, and increased E2F4 levels have no effect. These data are consistent with the notion that E2F1 is a regulator of chondrocyte differentiation.
Figures

References
-
- Alkema, M. J., N. M. van der Lugt, R. C. Bobeldijk, A. Berns, and M. van Lohuizen. 1995. Transformation of axial skeleton due to overexpression of bmi-1 in transgenic mice. Nature 374:724-727. - PubMed
-
- Alvarez, J., P. Sohn, X. Zeng, T. Doetschman, D. J. Robbins, and R. Serra. 2002. TGFbeta2 mediates the effects of hedgehog on hypertrophic differentiation and PTHrP expression. Development 129:1913-1924. - PubMed
-
- Amanullah, A., B. Hoffman, and D. A. Liebermann. 2000. Deregulated E2F-1 blocks terminal differentiation and loss of leukemogenicity of M1 myeloblastic leukemia cells without abrogating induction of p15(INK4B) and p16(INK4A). Blood 96:475-482. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
