Generation of neural crest-derived peripheral neurons and floor plate cells from mouse and primate embryonic stem cells
- PMID: 12724518
- PMCID: PMC156286
- DOI: 10.1073/pnas.1037282100
Generation of neural crest-derived peripheral neurons and floor plate cells from mouse and primate embryonic stem cells
Abstract
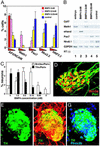
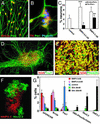
To understand the range of competence of embryonic stem (ES) cell-derived neural precursors, we have examined in vitro differentiation of mouse and primate ES cells into the dorsal- (neural crest) and ventralmost (floor plate) cells of the neural axis. Stromal cell-derived inducing activity (SDIA; accumulated on PA6 stromal cells) induces cocultured ES cells to differentiate into rostral CNS tissues containing both ventral and dorsal cells. Although early exposure of SDIA-treated ES cells to bone morphogenetic protein (BMP)4 suppresses neural differentiation and promotes epidermogenesis, late BMP4 exposure after the fourth day of coculture causes differentiation of neural crest cells and dorsalmost CNS cells, with autonomic system and sensory lineages induced preferentially by high and low BMP4 concentrations, respectively. In contrast, Sonic hedgehog (Shh) suppresses differentiation of neural crest lineages and promotes that of ventral CNS tissues such as motor neurons. Notably, high concentrations of Shh efficiently promote differentiation of HNF3beta(+) floor plate cells with axonal guidance activities. Thus, SDIA-treated ES cells generate naive precursors that have the competence of differentiating into the "full" dorsal-ventral range of neuroectodermal derivatives in response to patterning signals.
Figures


References
-
- Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, Nishikawa S-I, Sasai Y. Neuron. 2000;28:31–40. - PubMed
-
- Lee S-H, Lumelsky N, Studer L, Auerbach J M, McKay R D. Nat Biotechnol. 2000;18:675–679. - PubMed
-
- Wichterie H, Liberam I, Porter J A, Jessell T M. Cell. 2002;110:385–397. - PubMed
-
- Suemori H, Tada T, Torii R, Hosoi Y, Kobayashi K, Imahie H, Kondo Y, Iritani A, Nakatsuji N. Dev Dyn. 2001;222:273–279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

