MmpL8 is required for sulfolipid-1 biosynthesis and Mycobacterium tuberculosis virulence
- PMID: 12724526
- PMCID: PMC156336
- DOI: 10.1073/pnas.1030024100
MmpL8 is required for sulfolipid-1 biosynthesis and Mycobacterium tuberculosis virulence
Abstract
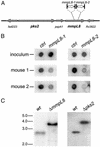
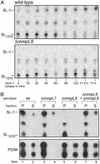
Mycobacterium tuberculosis, the causative agent of human tuberculosis, is unique among bacterial pathogens in that it displays a wide array of complex lipids and lipoglycans on its cell surface. One of the more remarkable lipids is a sulfated glycolipid, termed sulfolipid-1 (SL-1), which is thought to mediate specific host-pathogen interactions during infection. However, a direct role for SL-1 in M. tuberculosis virulence has not been established. Here we show that MmpL8, a member of a large family of predicted lipid transporters in M. tuberculosis, is required for SL-1 production. The accumulation of an SL-1 precursor, termed SL(1278), in mmpL8 mutant cells indicates that MmpL8 is necessary for an intermediate step in the SL-1 biosynthesis pathway. We use a novel fractionation procedure to demonstrate that SL-1 is present on the cell surface, whereas SL(1278) is found exclusively in more internal layers. Importantly, we show that mmpL8 mutants are attenuated for growth in a mouse model of tuberculosis. However, SL-1 per se is not required for establishing infection as pks2 mutants, which are defective in an earlier step in SL-1 biosynthesis, have no obvious growth defect. Thus, we hypothesize that either MmpL8 transports molecules in addition to SL-1 that mediate host-pathogen interactions or the accumulation of SL(1278) in mmpL8 mutant cells interferes with other pathways required for growth during the early stages of infection.
Figures
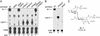
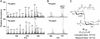

References
-
- Russell D G. Nat Rev Mol Cell Biol. 2001;2:569–577. - PubMed
-
- McKinney J D, Jacobs J, W R, Bloom B R. In: Emerging Infections. Fauci A, Krause R, editors. London: Academic; 1998. pp. 51–146.
-
- Glickman M S, Jacobs W R., Jr Cell. 2001;104:477–485. - PubMed
-
- Brennan P J, Nikaido H. Annu Rev Biochem. 1995;64:29–63. - PubMed
-
- Nikaido H, Kim S H, Rosenberg E Y. Mol Microbiol. 1993;8:1025–1030. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

