Unified two-metal mechanism of RNA synthesis and degradation by RNA polymerase
- PMID: 12727889
- PMCID: PMC156065
- DOI: 10.1093/emboj/cdg193
Unified two-metal mechanism of RNA synthesis and degradation by RNA polymerase
Abstract
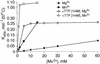
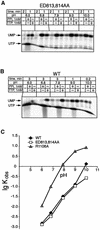
In DNA-dependent RNA polymerases, reactions of RNA synthesis and degradation are performed by the same active center (in contrast to DNA polymerases in which they are separate). We propose a unified catalytic mechanism for multisubunit RNA polymerases based on the analysis of its 3'-5' exonuclease reaction in the context of crystal structure. The active center involves a symmetrical pair of Mg(2+) ions that switch roles in synthesis and degradation. One ion is retained permanently and the other is recruited ad hoc for each act of catalysis. The weakly bound Mg(2+) is stabilized in the active center in different modes depending on the type of reaction: during synthesis by the beta,gamma-phosphates of the incoming substrate; and during hydrolysis by the phosphates of a non-base-paired nucleoside triphosphate. The latter mode defines a transient, non-specific nucleoside triphosphate-binding site adjacent to the active center, which may serve as a gateway for polymerization of substrates.
Figures



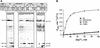

References
-
- Borukhov S. and Goldfarb,A. (1993) Recombinant Escherichia coli RNA polymerase: purification of individually overexpressed subunits and in vitro assembly. Protein Expr. Purif., 4, 503–511. - PubMed
-
- Borukhov S., Sagitov,V. and Goldfarb,A. (1993) Transcript cleavage factors from E.coli. Cell, 72, 459–466. - PubMed
-
- Brautigam C.A. and Steitz,T.A. (1998) Structural principles for the inhibition of the 3′–5′ exonuclease activity of Escherichia coli DNA polymerase I by phosphorothioates. J. Mol. Biol., 277, 363–377. - PubMed
-
- Cramer P., Bushnell,D.A. and Kornberg,R.D. (2001) Structural basis of transcription: RNA polymerase II at 2.8 Å resolution. Science, 292, 1863–1876. - PubMed
-
- Epshtein V., Mustaev,A., Markovtsov,V., Bereshchenko,O., Nikiforov,V. and Goldfarb,A. (2002) Swing-gate model of nucleotide entry into the RNA polymerase active center. Mol. Cell, 10, 623–634. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

