Action potentials in basal and oblique dendrites of rat neocortical pyramidal neurons
- PMID: 12730348
- PMCID: PMC2343022
- DOI: 10.1113/jphysiol.2002.033746
Action potentials in basal and oblique dendrites of rat neocortical pyramidal neurons
Abstract
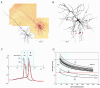
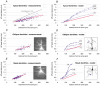
Basal and oblique dendrites comprise ~2/3 of the total excitable membrane in the mammalian cerebral cortex, yet they have never been probed with glass electrodes, and therefore their electrical properties and overall impact on synaptic processing are unknown. In the present study, fast multi-site voltage-sensitive dye imaging combined with somatic recording was used to provide a detailed description of the membrane potential transients in basal and oblique dendrites of pyramidal neurons during single and trains of action potentials (APs). The optical method allowed simultaneous measurements from several dendrites in the visual field up to 200 microm from the soma, thus providing a unique report on how an AP invades the entire dendritic tree. In contrast to apical dendrites, basal and oblique branches: (1) impose very little amplitude and time course modulation on backpropagating APs; (2) are strongly invaded by the somatic spike even when somatic firing rates reach 40 Hz (activity-independent backpropagation); and (3) do not exhibit signs of a 'calcium shoulder' on the falling phase of the AP. A compartmental model incorporating AP peak latencies and half-widths obtained from the apical, oblique and basal dendrites indicates that the specific intracellular resistance (Ri) is less than 100 omicron cm. The combined experimental and modelling results also provide evidence that all synaptic locations along basal and oblique dendrites, situated within 200 microm from the soma, experience strong and near-simultaneous (latency < 1 ms) voltage transients during somatic firing. The cell body, axon hillock and basal dendritic compartments achieve unique synchronization during each AP. Therefore, with respect to a retrograde signal (AP), basal and proximal oblique dendrites should be considered as an integral part of the axo-somatic compartment.
Figures



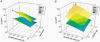
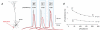


References
-
- Anderson JC, Binzegger T, Kahana O, Martin KA, Segev I. Dendritic asymmetry cannot account for directional responses of neurons in visual cortex. Nat Neurosci. 1999;2:820–824. - PubMed
-
- Antic S, Major G, Zecevic D. Fast optical recordings of membrane potential changes from dendrites of pyramidal neurons. J Neurophysiol. 1999;82:1615–1621. - PubMed
-
- Archie KA, Mel BW. A model for intradendritic computation of binocular disparity. Nat Neurosci. 2000;3:54–63. - PubMed
-
- Baer SM, Rinzel J. Propagation of dendritic spikes mediated by excitable spines: a continuum theory. J Neurophysiol. 1991;65:874–890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

