Tuning activation of the AMPA-sensitive GluR2 ion channel by genetic adjustment of agonist-induced conformational changes
- PMID: 12730367
- PMCID: PMC156270
- DOI: 10.1073/pnas.1037393100
Tuning activation of the AMPA-sensitive GluR2 ion channel by genetic adjustment of agonist-induced conformational changes
Abstract
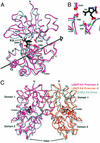
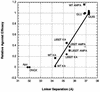
The (S)-2-amino-3-(3-hydroxy-5-methyl-4-isoxazole) propionic acid (AMPA) receptor discriminates between agonists in terms of binding and channel gating; AMPA is a high-affinity full agonist, whereas kainate is a low-affinity partial agonist. Although there is extensive literature on the functional characterization of partial agonist activity in ion channels, structure-based mechanisms are scarce. Here we investigate the role of Leu-650, a binding cleft residue conserved among AMPA receptors, in maintaining agonist specificity and regulating agonist binding and channel gating by using physiological, x-ray crystallographic, and biochemical techniques. Changing Leu-650 to Thr yields a receptor that responds more potently and efficaciously to kainate and less potently and efficaciously to AMPA relative to the WT receptor. Crystal structures of the Leu-650 to Thr mutant reveal an increase in domain closure in the kainate-bound state and a partially closed and a fully closed conformation in the AMPA-bound form. Our results indicate that agonists can induce a range of conformations in the GluR2 ligand-binding core and that domain closure is directly correlated to channel activation. The partially closed, AMPA-bound conformation of the L650T mutant likely captures the structure of an agonist-bound, inactive state of the receptor. Together with previously solved structures, we have determined a mechanism of agonist binding and subsequent conformational rearrangements.
Figures

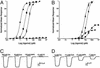

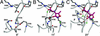
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

