Prokaryotic metabolic activity and community structure in Antarctic continental shelf sediments
- PMID: 12732510
- PMCID: PMC154502
- DOI: 10.1128/AEM.69.5.2448-2462.2003
Prokaryotic metabolic activity and community structure in Antarctic continental shelf sediments
Abstract
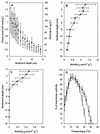
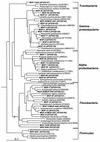
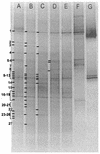
The prokaryote community activity and structural characteristics within marine sediment sampled across a continental shelf area located off eastern Antarctica (66 degrees S, 143 degrees E; depth range, 709 to 964 m) were studied. Correlations were found between microbial biomass and aminopeptidase and chitinase rates, which were used as proxies for microbial activity. Biomass and activity were maximal within the 0- to 3-cm depth range and declined rapidly with sediment depths below 5 cm. Most-probable-number counting using a dilute carbohydrate-containing medium recovered 1.7 to 3.8% of the sediment total bacterial count, with mostly facultatively anaerobic psychrophiles cultured. The median optimal growth temperature for the sediment isolates was 15 degrees C. Many of the isolates identified belonged to genera characteristic of deep-sea habitats, although most appear to be novel species. Phospholipid fatty acid (PLFA) and isoprenoid glycerol dialkyl glycerol tetraether analyses indicated that the samples contained lipid components typical of marine sediments, with profiles varying little between samples at the same depth; however, significant differences in PLFA profiles were found between depths of 0 to 1 cm and 13 to 15 cm, reflecting the presence of a different microbial community. Denaturing gradient gel electrophoresis (DGGE) analysis of amplified bacterial 16S rRNA genes revealed that between samples and across sediment core depths of 1 to 4 cm, the community structure appeared homogenous; however, principal-component analysis of DGGE patterns revealed that at greater sediment depths, successional shifts in community structure were evident. Sequencing of DGGE bands and rRNA probe hybridization analysis revealed that the major community members belonged to delta proteobacteria, putative sulfide oxidizers of the gamma proteobacteria, Flavobacteria, Planctomycetales, and Archaea. rRNA hybridization analyses also indicated that these groups were present at similar levels in the top layer across the shelf region.
Figures
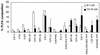



References
-
- Belanger, C., B. DesRosiers, and K. Lee. 1997. Microbial extracellular enzyme activity in marine sediments—extreme pH to terminate reaction and sample storage. Aquat. Microb. Ecol. 13:187-196.
-
- Bindoff, N. L., G. D. Williams, and I. Allison. 2000. Sea-ice growth and water mass modification in the Mertz Glacier Polynya during winter. Ann. Glaciol. 33:399-406.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

