The ars detoxification system is advantageous but not required for As(V) respiration by the genetically tractable Shewanella species strain ANA-3
- PMID: 12732551
- PMCID: PMC154534
- DOI: 10.1128/AEM.69.5.2800-2809.2003
The ars detoxification system is advantageous but not required for As(V) respiration by the genetically tractable Shewanella species strain ANA-3
Abstract
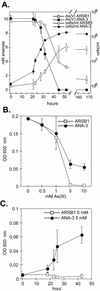
Arsenate [As(V); HAsO(4)(2-)] respiration by bacteria is poorly understood at the molecular level largely due to a paucity of genetically tractable organisms with this metabolic capability. We report here the isolation of a new As(V)-respiring strain (ANA-3) that is phylogenetically related to members of the genus Shewanella and that also provides a useful model system with which to explore the molecular basis of As(V) respiration. This gram-negative strain stoichiometrically couples the oxidation of lactate to acetate with the reduction of As(V) to arsenite [As(III); HAsO(2)]. The generation time and lactate molar growth yield (Y(lactate)) are 2.8 h and 10.0 g of cells mol of lactate(-1), respectively, when it is grown anaerobically on lactate and As(V). ANA-3 uses a wide variety of terminal electron acceptors, including oxygen, soluble ferric iron, oxides of iron and manganese, nitrate, fumarate, the humic acid functional analog 2,6-anthraquinone disulfonate, and thiosulfate. ANA-3 also reduces As(V) to As(III) in the presence of oxygen and resists high concentrations of As(III) (up to 10 mM) when grown under either aerobic or anaerobic conditions. ANA-3 possesses an ars operon (arsDABC) that allows it to resist high levels of As(III); this operon also confers resistance to the As-sensitive strains Shewanella oneidensis MR-1 and Escherichia coli AW3110. When the gene encoding the As(III) efflux pump, arsB, is inactivated in ANA-3 by a polar mutation that also eliminates the expression of arsC, which encodes an As(V) reductase, the resulting As(III)-sensitive strain still respires As(V); however, the generation time and the Y(lactate) value are two- and threefold lower, respectively, than those of the wild type. These results suggest that ArsB and ArsC may be useful for As(V)-respiring bacteria in environments where As concentrations are high, but that neither is required for respiration.
Figures



References
-
- Ahmann, D., L. R. Krumholz, H. F. Hemond, D. R. Lovley, and F. M. Morel. 1997. Microbial mobilization of arsenic from sediments of the Aberjona watershed. Environ. Sci. Technol. 31:2923-2930.
-
- Armienta, M. A., G. Villasenor, R. Rodriguez, L. K. Ongley, and H. Mango. 2001. The role of arsenic-bearing rocks in groundwater pollution at Zimapan Valley, Mexico. Environ. Geol. 40:571-581.
-
- Ausubel, F. M. 1992. Short protocols in molecular biology, 2nd ed.: a compendium of methods from current protocols in molecular biology. John Wiley & Sons, Inc./Green Publishing Associates, New York, N.Y.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

